You are here: Home > Medical Countermeasures Database > Reactive Skin Decontamination Lotion (RSDL)
Reactive Skin Decontamination Lotion (RSDL) -
Medical Countermeasures Database
1. Name of Chemical Defense therapeutic agent/device
Reactive Skin Decontamination Lotion (RSDL)
2. Chemical Defense therapeutic area(s)
— including key possible uses-
[2022 publication. Italics added during CHEMM annotation.]
Abstract. Due to threats posed by chemical warfare agents (CWAs) and accidents with toxic industrial chemicals (TICs), the need for highly effective skin decontamination remains relevant. Reactive Skin Decontamination Lotion (RSDL), composed of Dekon 139 and 2,3-butanedione monoxime, has been shown highly effective against CWAs and TICs. This systematic review compares RSDL efficacy to other decontaminating agents. Online search engines PubMed, Web of Science, and Embase were explored and all literatures containing quantitative data, comparing RSDL to other decontaminating agents, investigated. Year of publication, type of study (in vitro or in vivo), model (animal or human), toxin tested, and result of each relevant article were recorded. In total, 15 relevant papers, comprising a total of 18 experimental models, were identified. Nine studies concluded that RSDL was the most effective decontaminant tested against the toxin of interest. Four studies concluded that RSDL was not the most effective decontaminant tested against the toxin of interest. The remaining five studies concluded RSDL displayed similar efficacy to at least one of the other decontaminating agents tested against the toxin of interest. There is substantial evidence supporting the efficacy of RSDL as a decontaminating agent. However, there remains to be insufficient data on this important topic, and limitations on the usefulness of current data, when applied to the broad array of potential exposures.
Feschuk, A.M., Law, R.M., Maibach, H.I. (2022). A Review of Reactive Skin Decontamination Lotion Efficacy. In: Feschuk, A.M., Law, R.M., Maibach, H.I. (eds) Dermal Absorption and Decontamination. Springer, Cham. https://doi.org/10.1007/978-3-031-09222-0_8
-
[2022 publication. Italics added during CHEMM annotation.]
Treatment/ Management. The most important treatment is to terminate the patient's exposure by removing them from the contaminated environment and performing decontamination. Decontamination of the patient should always be performed before further medical treatment if possible. Some nerve agents may remain in the skin and continue to cause symptoms via a depot effect. Patients can be decontaminated by washing the affected areas with a 0.5% hypochlorite solution or with soap and clean water. However, the most effective method of decontamination of patients exposed to nerve agents is Reactive Skin Decontamination Lotion (RSDL), which should be used if available. The ingredients of Reactive Skin Decontamination Lotion sequester, retain, and neutralize organophosphate chemical warfare agents. Patients with large areas of dermal exposure will require numerous Reactive Skin Decontamination Lotion sponges to be effectively decontaminated.
Hayoun MA, Smith ME, Ausman C, et al. Toxicology, V-Series Nerve Agents. [Updated 2022 Sep 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441997/
-
[2022 publication. Italics added during CHEMM annotation.].
If available and under the right circumstances, reactive skin decontamination lotion (RSDL) is an option as an adjunct to aqueous decontamination. RSDL consists of multiple components, including Dekon 139 and 2,3-butanedione monoxime (DAM), which chelate and inactive [sic] several military-grade chemical agents. RSDL has been formulated to remove and neutralize several agents, including organophosphates, vesicants such as mustard gas, and can be used to decontaminate T-2, a weaponized fungal toxin. It comes packaged with a pre-treated sponge. One sponge is not sufficient to completely decontaminate an individual and is intended for use on the face, neck, hands, and the inner surface of a respirator. RSDL is more effective than diluted sodium hypochlorite and soapy water alone.[8] Johnston GM, Wills BK. Chemical Decontamination. [Updated 2022 Jun 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538161/
-
[2022 publication. Italics added during CHEMM annotation.]
Abstract. Although skin decontamination has been studied since WWI, there has yet been an optimal protocol for determining in vitro skin decontamination efficacy due to the complexity of percutaneous absorption. Here we explore methods of quantifying decontamination through the parameters of percutaneous absorption in different models: humans, animals, in vitro assays, and computer modeling, with humans being an ideal model, followed by rhesus monkeys, weanling pigs, hairless guinea pigs, and hairless rats. In vitro assays are also useful, but skin type/thickness, receptor fluid, etc. must be chosen carefully as these can influence absorption. Computer modeling provides another way to quantify percutaneous absorption when sufficient input data becomes available. Reactive skin decontamination lotion (RSDL) is considered a gold standard for decontamination and is used by the military to decontaminate chemical warfare agents. Although there is evidence that supports RSDL superiority over other methods of decontamination, lack of in vivo human studies and diversity in types of compounds tested remain a data limitation. An ideal decontamination method is applying the contaminant on the test model and measuring the mass absorbed when a decontaminant is used compared to when no decontaminant is used. A perfect decontaminant would result in no contaminant absorption.
Tran, T., Maibach, H.I. (2022). Toward a Harmonized Protocol for Quantifying In Vitro Human Skin Decontamination Efficacy. In: Feschuk, A.M., Law, R.M., Maibach, H.I. (eds) Dermal Absorption and Decontamination. Springer, Cham. https://link.springer.com/chapter/10.1007/978-3-031-09222-0_2
-
[2022 publication. Italics added during CHEMM annotation.]
“The following agents may be used for spot decontamination [3,63,64]:
Reactive Skin Decontamination Lotion (RSDL) – RSDL is specifically formulated to neutralize the toxicity of the liquid nerve agent VX and blister agents such as sulfur mustard and Lewisite. It acts within seconds of being applied to the skin and is the preferred spot decontamination method for victims of a chemical attack [63,65]. The lotion is packaged on a foam applicator inside a single use pouch. The lotion is applied within three minutes of contamination and rubbed gently on the skin for two minutes, and the nontoxic residue is washed away at a later time (picture 1). Although reasonable to perform, application of RSDL to wounds is considered off label use. Application of RSDL to the eyes is not recommended.Other topical absorbents – If RSDL is not available, porous material such as activated charcoal, Fuller's earth, clay, tissue paper, flour, or bread, although less effective, may be applied to areas of contaminated skin to adsorb agent followed by irrigation. If an adsorbent is not available, irrigation alone should be performed.”
Madsen,. Chemical terrorism: Rapid recognition and initial medical management. UpToDate.https://www.uptodate.com/contents/chemical-terrorism-rapid-recognition-and-initial-medical-management (“This topic last updated: May 24, 2022”)
-
[2021 publication. Italics added during CHEMM annotation.]
Excerpts
- Use Reactive Skin Decontaminant Lotion (RSDL) if available, according to the instructions of use, on all areas of skin that are suspected of being contaminated. RSDL is highly effective at absorbing G- and V-series nerve agents, as well as FGAs, from the skin. RSDL solvates and neutralizes G- and V-series nerve agents on application and scrubbing. Of special note, RSDL does not neutralize FGAs efficiently. Accordingly, it is important that RSDL be removed from the skin using the included sponge, or a cloth/paper towel to “lift and shift” agent and or degradation products off the skin. The lift and shift action of RSDL is especially critical to its efficacy against FGAs;
- Water effluent and any other decontamination formulation including RSDL generated during the decontamination process should be contained and treated as a hazardous waste. FGAs are very stable in water;
- RSDL and RSDL effluent must never be exposed to or come in contact with bleach solution/powder, super tropical bleach (STB) or high-test hypochlorite (HTH), which contain the strong oxidizing chemical calcium hypochlorite. RSDL may become very hot and combust when exposed to these chemicals; Duncan S, Mikler J, Jackson Lepage C, Clewley R, Boulay Greene H. Responding to an incident involving organophosphorus nerve agents Safety advisory and guidance. Defence Research and Development. Canada Reference Document DRDC-RDDC-2021-D106. September 2021. https://cradpdf.drdc-rddc.gc.ca/PDFS/unc366/p813529_A1b.pdf
-
[2021 publication. Italics added during CHEMM annotation.]
Nuclophilic [sic] Substitution: Rapid nucleophilic decon is efficient for decontamination but non appropriate for skin [80]. But, Reactive Skin Decontamination Lotion (RSDL), is based on a mechanism chemically similar to hydrolysis, but at milder pH. Active ingredient, a nucleophilic compound (2,3 butanedione monoxime), also called, Diacetyl Monoxime (DAM) is dissolved in a glycol solvent which dissolves most agents and allow them to be rinsed off with water. Decontamination must be initiated within 5 to 10 minutes to be high efficient [81]. RSDL destroys CWA without corrosive effects, and conteract the inflammator process, especially against sulfur mustard [35]. RSDL, a skin decontaminant FDA approved (2002), [82]. This viscous yellow lotion impregnated in a polymer sponge (pH 10.5) is water soluble, therefore easy to eliminate after decon, and presented in individual packets ready to use (5 year shelf life). The sponge is applied in a overlapping circular motion to skin, requiring at least 2 minutes contact to achieve neutralization of agents (nerve agents, pesticides). For safety DAM, active ingredient of RSDL may be percutaneous absorbed (MW 101.1 ; log P (octanol-water) 1.740) [83], The acute toxic effects of DAM, observed after injection to rats and rabbits appears due to the compound itself [84]. RSDL is not approved for eyes, or in wounds [82]. Safety precautions are recommended for RSDL: short time application (< 6 hours), rinsing after decontamination and respect the incompatibility with solid bleaching.
Roul A, Maibach HHI (2020) Skin Decontamination 2021. Emerg Med Inves 5: 10105. DOI: https://doi.org/10.29011/2475-5605.010105
https://www.gavinpublishers.com/article/view/skin-decontamination-2021 -
“The RSDL Kit is the only decontaminant that is intended to remove and/or neutralize chemical warfare agents, including tabun, sarin, soman, cyclohexyl sarin, VR, VX, mustard gas and T-2 toxin, from the skin in a single step.”
And
“Decontamination occurs by physical removal of the chemical warfare agent from the skin or by chemical neutralization.
The chemical neutralization occurs by a reaction mechanism known as nucleophilic substitution.
The reactions occurring between the lotion and various chemical warfare agents have been widely studied both in vitro and in vivo, providing data to demonstrate that the resulting reaction products can be rinsed off when operational conditions permit.”
https://www.rsdl.com/resources/ (Emergent BioSolutions Inc. web site “for professional audiences in the U.S.”)
-
[2021 publication. Italics added during CHEMM annotation.]
“The two decontaminant systems that are currently mostly in use for skin decontamination are Fuller's Earth (FE) and Reactive Skin Decontaminant Lotion (RSDL). Both, FE and RSDL, were designed for use as emergency decontaminant systems for skin decontamination in the field. FE is a natural substance containing silica of aluminum and magnesium and has very high adsorption properties. In addition, FE contains small amounts of calcite, dolomite and quartz. The adsorbing powder is fielded for military needs in many countries for use after dermal exposure. Previous investigations have shown that decontamination by FE is effective if it is performed within a short time after exposure [11].”
Dachir S, Cohen M, Buch H, Kadar T. Skin decontamination efficacy of sulfur mustard and VX in the pig model: A comparison between Fuller's earth and RSDL. Chem Biol Interact. 2021 Feb 25;336:109393. https://pubmed.ncbi.nlm.nih.gov/33508307/ [PubMed Citation]
-
[2020 publication. Italics added during CHEMM annotation.]
Abstract. Awareness and concern over the occupational health and safety of first responders to biological threat and other hazardous exposures has grown. Law enforcement personnel play an important role in the response to such events and may even be the first on the scene to hazardous exposures. Front line police entering a property and expecting to find drugs and weapons may also unexpectedly find biological or chemical agents. In the case of a pandemic like COVID-19, they may be exposed to virus in their ordinary duties. We argue that the risk of exposure is increasing, and will continue to increase, driven by advances in science and biology which makes chemical and biological agents more accessible to a wide range of actors. In addition, serious epidemics of newly emerged infections are increasing in frequency. Although the level of risk to police will vary depending on the exposure, the uniformed officers at the front line may be at highest risk because of a higher likelihood of being unprotected when they encounter biothreats. Planning focuses on response to known events by well-trained and well-equipped HAZMAT (hazardous materials) teams. Better preparedness is required for unexpected exposure of front-line police. This includes expanded training and design of regular uniforms to reduce exposure, provision of personal protective equipment (PPE) kits which include disinfectant wipes, chemical wipes and biosensors. As the use of chemical and biological weapons by nefarious actors increases, these changes may become a necessity to protect the occupational health and safety of police.
Excerpt:
Reactive skin decontamination lotion (RSDL) or wipes should be considered as protection for front line police. There is some evidence that RSDL is effective for chemical decontamination during the military and civilian emergences (41). These contain Dekon 139 which can decontaminate nerve agents and other chemicals (42). These are not routinely provided to front line police as part of their PPE kits, with cost being the major barrier. However, increasing likelihood of exposure to chemical agents will shift the cost-effectiveness estimates of routinely providing RSDL wipes to officers. Disinfectant wipes or lotion can also be considered in the PPE kit of officers to clean inadvertent biological contaminants. During a pandemic such as COVID-19, hand sanitizer could be made available in every patrol car, and police provided with disinfectant wipes and masks.MacIntyre, C.R., Chughtai, A.A., Bhattacharjee, S., Kunasekaran, M.P. and Engalls, T., 2020. Risk mitigation of inadvertent exposure to biothreats to front line law enforcement. Global Biosecurity, 2(1), p.None.https://jglobalbiosecurity.com/articles/10.31646/gbio.59/
-
[2016 publication. Italics added during CHEMM annotation.]
Excerpts:
- Various decontamination products are available on the market. Corrosive decontaminants, e.g., sodium hypochlorite (bleach), are highly effective but due to their irritating and damaging effects on skin are not recommended for skin decontamination.
- At present, Reactive Skin Decontamination Lotion (RSDL) appears to be an appropriate decontaminant for humans in order to perform spot decontamination. RSDL was introduced in several military forces as a skin decontamination product to remove or neutralize chemical warfare agents, T-2 toxin and many pesticide related chemicals from the skin. It received a license from the US Food and Drug Administration (FDA) and has received a European CE Mark and an Australian TGA license http://www.rsdl.com/skin-decontamination/rsdl/. However, RSDL is not approved for use in the eyes or in wounds, as it may impair wound healing. 23
- In conclusion, a decontaminant for skin, eye, wound and mucous membrane decontamination is not available at present. If eyes are suspected to be contaminated, extensive rinsing should be performed with, ideally, physiological solutions, e.g. 0.9% saline solution, but sterile or at least clean water could be used. However, care should be taken in order to prevent eye damage due to vigorous procedures. Equally, wounds should be extensively rinsed with sterile physiological solutions. Here, however, a thorough balance may be necessary in order to prevent secondary damage due to exposure to great amounts of fluid in large wounds. In conclusion, effective personnel decontamination combines an immediate spot decontamination, e.g., by RSDL, followed by undressing and whole body decontamination by showering with plenty of water or soapy water at ambient temperature.
-
Thiermann H, Aurbek N, and Worek F, CHAPTER 1: Treatment
of Nerve Agent Poisoning , in Chemical Warfare Toxicology:
Volume 2: Management of Poisoning, 2016, pp. 1-42.
https://pubs.rsc.org/en/content/chapterhtml/2016/bk9781782628033-00001
-
[2016 publication. Italics added during CHEMM annotation.]
Abstract: The goal of this study was to provide quantitative efficacy information relevant to technical and forensic decontamination that may assist safety officers in mitigating health hazards to personnel and minimizing the potential transfer of chemicals by cross-contamination from a chemical incident scene. To achieve this goal, decontamination solutions were evaluated using representative contact times of personal protective equipment (PPE) and related materials with the chemical contaminant at operationally relevant decontamination dwell times. The study included four toxic industrial chemicals (TICs) and two chemical warfare agent (CWA) surrogates, eight decontamination solutions and seven PPE-related materials that would transition through a personnel decontamination line. Measured neutralization efficacies following a 2.0-min dwell time varied strongly by chemical with no/very minimal efficacy observed for decontaminants against materials contaminated with nitrobenzene, chlordane, and phenol. Higher efficacies up to 60% were observed for full strength bleach, RSDL® and EasyDECON® DF200 products against malathion, carbaryl, and 2-chloroethyl ethyl sulfide. Other decontamination solutions like detergent and water, 10× diluted bleach, and pH-amended bleach were found to be non-efficacious (less than 20%) against any of the chemicals. The short dwell time and limited amount of decontaminant on the contaminated surface limits the expected efficacy. Therefore, the contribution of neutralization to the decontamination process while responders are preparing to doff personal protective equipment may be limited.
Oudejans L, O’Kelly J, Evans AS, Wyrzykowska-Ceradini B, Abderrahmane Touati A, Dennis Tabor D, Snyder EG,
Decontamination of personal protective equipment and related materials contaminated with toxic industrial chemicals and chemical warfare agent surrogates,
Journal of Environmental Chemical Engineering, Volume 4, Issue 3,
2016, Pages 2745-2753,
ISSN 2213-3437,
https://www.sciencedirect.com/science/article/pii/S2213343716301920 (Not in PubMed)
3. Evidence-based medicine for Chemical Defense
— including efficacy and safetyA. Summary
Mechanism of action
-
How does the RSDL Kit work? Decontamination occurs by physical removal of the chemical warfare agent from the skin or by chemical neutralization. The chemical neutralization occurs by a reaction mechanism known as nucleophilic substitution. The reactions occurring between the lotion and various chemical warfare agents have been widely studied both in vitro and in vivo, providing data to demonstrate that the resulting reaction products can be rinsed off when operational conditions permit.
https://www.rsdl.com/resources/ (Emergent BioSolutions Inc. web site “for professional audiences in the U.S.”)
-
RSDL contains Dekon 139 and a small amount of 2,3-butanedione monoxime (DAM). These compounds are dissolved in a solvent composed of polyethylene glycol monomethyl ether (MPEG) and water. This solvent system is particularly important as it promotes the decontamination reaction by actively desorbing, retaining and sequestering the chemical agent, while the active ingredient (Dekon 139) chemically reacts with, and rapidly neutralizes the vesicant chemical or the organophophosphorous nerve agent. This reaction starts immediately and neutralization is usually complete within two minutes.
This text used to be in the online content for: https://www.rsdl.com/resources/ (Emergent BioSolutions Inc. web site “for professional audiences in the U.S.”)
-
Reactive Skin Decontamination Lotion (RSDL), composed of Dekon 139 and 2,3-butanedione monoxime, has been shown highly effective against CWAs and TICs.
Feschuk AM, Law RM, Maibach HI. Comparative efficacy of Reactive Skin Decontamination Lotion (RSDL): A systematic review. Toxicol Lett. 2021 Oct 1;349:109-114. https://pubmed.ncbi.nlm.nih.gov/34147606/ [PubMed Citation]
-
[2021 publication. Italics added during CHEMM annotation.]
Abstract. Aim of the study: Following percutaneous exposure to the nerve agent VX, the remaining intact agent within the skin after decontamination is of great concern. Consequently, this leads to prolonged agent release to the blood circulation resulting in sustained intoxication, which may complicate the medical management. The decontamination procedure used should therefore possess the ability for agent removal both on and within the skin. The efficacy of three decontamination procedures was evaluated by measuring VX and the primary degradation product ethyl methyl phosphonic acid (EMPA) penetrated through human skin and the amount remaining within the skin. Materials and methods: Decontamination was initiated 5 min post-exposure to VX on human dermatomed skin. Experiments were conducted using an in vitro skin penetration model and the amount remaining within the skin was determined by combining the tape-stripping technique and acetylcholinesterase activity measurements. Results: In control experiments without decontamination, higher amounts of VX were recovered in the deeper layers of skin compared to EMPA, which was primarily located in the stratum corneum. Both Reactive Skin Decontamination Lotion (RSDL) and the RSDL training kit (TRSDL) significantly reduced the amount of VX within the skin and decreased the penetration through the skin. However, the degradation ability of RSDL was demonstrated to be beneficial by the reduction of intact agents remaining in the skin compared to TRSDL without agent degradation capability. Soapy water decontamination caused a "wash-in" effect of VX with decreased agent amounts within stratum corneum but increased the amount VX penetrated through the skin. Conclusion: Efficient skin decontamination of VX requires skin decontaminants reaching deeper layers of the skin, and that both absorption and degradation properties are important. In addition, the "wash-in" effect by using soapy water may enhance VX release to the blood circulation.
Thors L, Wigenstam E, Qvarnström J, Bucht A. Efficient agent degradation within skin is important for decontamination of percutaneously exposed VX. Cutan Ocul Toxicol. 2021 Jun;40(2):95-102.
https://pubmed.ncbi.nlm.nih.gov/33759679/
Summary of clinical and non-clinical studies
[2021 publication. Italics added during CHEMM annotation.]
Abstract. Due to threats posed by chemical warfare agents (CWAs) and accidents with toxic industrial chemicals (TICs), the need for highly effective skin decontamination remains relevant. Reactive Skin Decontamination Lotion (RSDL), composed of Dekon 139 and 2,3-butanedione monoxime, has been shown highly effective against CWAs and TICs. This systematic review compares RSDL efficacy to other decontaminating agents. Online search engines PubMed, Web of Science, and Embase were explored and all literatures containing quantitative data, comparing RSDL to other decontaminating agents, investigated. Year of publication, type of study (in vitro or in vivo), model (animal or human), toxin tested, and result of each relevant article were recorded. In total, 15 relevant papers, comprising a total of 18 experimental models, were identified. Nine studies concluded that RSDL was the most effective decontaminant tested against the toxin of interest. Four studies concluded that RSDL was not the most effective decontaminant tested against the toxin of interest. The remaining five studies concluded RSDL displayed similar efficacy to at least one of the other decontaminating agents tested against the toxin of interest. There is substantial evidence supporting the efficacy of RSDL as a decontaminating agent. However, there remains to be insufficient data on this important topic, and limitations on the usefulness of current data, when applied to the broad array of potential exposures.
Feschuk AM, Law RM, Maibach HI. Comparative efficacy of Reactive
Skin Decontamination Lotion (RSDL): A systematic review. Toxicol
Lett. 2021 Oct 1;349:109-114. doi: 10.1016/j.toxlet.2021.06.010.
Epub 2021 Jun 17. PMID: 34147606.
https://pubmed.ncbi.nlm.nih.gov/34147606/ [PubMed Citation]
Note: The same text (the abstract noted above) appears in:
Feschuk, A.M., Law, R.M., Maibach, H.I. (2022). A Review of
Reactive Skin Decontamination Lotion Efficacy. In: Feschuk,
A.M., Law, R.M., Maibach, H.I. (eds) Dermal Absorption and
Decontamination. Springer, Cham.
https://link.springer.com/chapter/10.1007/978-3-031-09222-0_8
B. Link to clinical studies
Clinical reviews
-
Abstract. Rapid decontamination of the skin is the single most important action to prevent dermal absorption of chemical contaminants in persons exposed to chemical warfare agents (CWA) and toxic industrial chemicals (TICs) as a result of accidental or intentional release. Chemicals on the skin may be removed by mechanical means through the use of dry sorbents or water. Recent interest in decontamination systems which both partition contaminants away from the skin and actively neutralize the chemical has led to the development of several reactive decontamination solutions. This article will review the recently FDA-approved Reactive Skin Decontamination Lotion (RSDL) and will summarize the toxicity and efficacy studies conducted to date. Evidence of RSDL's superior performance against vesicant and organophosphorus chemical warfare agents compared to water, bleach, and dry sorbents, suggests that RSDL may have a role in mass human exposure chemical decontamination in both the military and civilian arenas (Class IV).
-
Skin Irritation (Cumulative). RSDL. 21-Day Cumulative (Occluded) Irritation Study in Humans. 0.05. ml on 1 cm sponge – topical application. RSDL was significantly less irritating the SLS positive control and significantly more irritating than the saline negative control. Results concluded that RSDL had a moderate potential for mild cumulative. Potential in normal use.
RSDL. 21-Day Cumulative (Occluded) Irritation Study in Humans. 0.05. ml on 1 cm sponge – topical application. RSDL was significantly less irritating the SLS positive control and significantly more irritating than the saline negative control. Results concluded that RSDL had a moderate potential for mild cumulative. Potential in normal use.
RSDL® (Reactive Skin Decontamination Lotion Kit). Safety Data Sheet 2017.
https://www.qckslvr.com/Images/quicksilveranalytics/sds/_T__RSDL_-_Emergent_PN_10051.pdf(This 2017 Safety Data Sheet is noted but not linked to in Emergent BioSolutions Inc. RSDL Consumer website: https://rsdlready.com/ -
Skin Sensitization.
RSDL. Human Repeat Insult Patch Test. RSDL was concluded to be non-sensitizing under the conditions of the study.
RSDL® (Reactive Skin Decontamination Lotion Kit). Safety Data Sheet 2017.
https://www.qckslvr.com/Images/quicksilveranalytics/sds/_T__RSDL_-_Emergent_PN_10051.pdf(This 2017 Safety Data Sheet is noted but not linked to in Emergent BioSolutions Inc. RSDL Consumer website: https://rsdlready.com/ -
Phototoxicity and Photoallergenicity.
RSDL. Evaluation of Phototoxicity and Photoallergenicity in Humans. 0.05 ml on 1 cm diameter sponge applied paraspinal. Under the conditions of the study there was no evidence of cutaneous RSDL phototoxicity or photoallergy.
RSDL® (Reactive Skin Decontamination Lotion Kit). Safety Data Sheet 2017.
https://www.qckslvr.com/Images/quicksilveranalytics/sds/_T__RSDL_-_Emergent_PN_10051.pdf(This 2017 Safety Data Sheet is noted but not linked to in Emergent BioSolutions Inc. RSDL Consumer website: https://rsdlready.com/[Human Hair Locks.] Abstract. Chemical warfare agents are an actual threat and victims' decontamination is a main concern when mass exposure occurs. Skin decontamination with current protocols has been widely documented, as well as surface decontamination. However, considering hair ability to trap chemicals in vapour phase, we investigated hair decontamination after exposure to sulphur mustard simulants methyl salicylate and 2-chloroethyl ethyl sulphide. Four decontamination protocols were tested on hair, combining showering and emergency decontamination (use of Fuller's earth or Reactive Skin Decontamination Lotion RSDL®). Both simulants were recovered from hair after treatment, but contents were significantly reduced (42-85% content allowance). Showering alone was the least efficient protocol. Concerning 2-chloroethyl ethyl sulphide, protocols did not display significant differences in decontamination efficacy. For MeS, use of emergency decontaminants significantly increased showering efficacy (10-20% rise), underlining their usefulness before thorough decontamination. Our results highlighted the need to extensively decontaminate hair after chemical exposure. Residual amounts after decontamination are challenging, as their release from hair could lead to health issues.
Spiandore M, Piram A, Lacoste A, Prevost P, Maloni P, Torre F, Asia L, Josse D, Doumenq P. Efficacy of scalp hair decontamination following exposure to vapours of sulphur mustard simulants 2-chloroethyl ethyl sulphide and methyl salicylate.Chem Biol Interact. 2017; 267:74-79. [PubMed Citation]
[Human Hair Locks.] Abstract. In this work, our goals were to establish whether hair decontamination by showering one hour post-exposure to the highly toxic organophosphate nerve agent VX was effective, whether it required the addition of a detergent to water and, if it could be improved by using the adsorbent Fuller's Earth (FE) or the Reactive Skin Decontamination Lotion (RSDL) 30 min prior to showering. Hair exposure to VX and decontamination was performed by using an in vitro model. Hair showering led to 72% reduction of contamination. Addition of detergent to water slightly increased the decontamination effectiveness. Hair treatment with FE or RSDL improved the decontamination rate. Combination of FE use and showering, which yielded a decontamination factor of 41, was demonstrated to be the most effective hair decontamination procedure. Hair wiping after showering was shown to contribute to hair decontamination. Altogether, our results highlighted the importance of considering hair decontamination as an important part of body surface decontamination protocols.
Josse D, Wartelle J, Cruz C. Showering effectiveness for human hair decontamination of the nerve agent VX. Chem Biol Interact. 2015; 232:94-100. [PubMed citation]
[Human Hair Locks.] Excerpt: In vitro studies of the efficacy of hair decontamination following exposure to VX16 and the sulphur mustard simulants methyl salicylate and 2-chloroethyl ethyl sulphide19 revealed that showering alone was the least effective decontamination protocol, whereas the application of Fullers Earth (FE) or Reactive Skin Decontamination Lotion (RSDL) prior to showering substantially improved decontamination efficacy up to 45 min post exposure. The same experiments also showed significant persistence of VX and methyl salicylate (MeS) in hair post-decontamination.
Collins, S., James, T., Southworth, F. et al. Human volunteer study of the decontamination of chemically contaminated hair and the consequences for systemic exposure. Sci Rep 10, 20822 (2020).
https://www.nature.com/articles/s41598-020-77930-1
And
Collins, S., James, T., Southworth, F. et al. Author Correction: Human volunteer study of the decontamination of chemically contaminated hair and the consequences for systemic exposure. Sci Rep 11, 9070 (2021). (“This Article contains errors in Figure 2.”)
https://www.nature.com/articles/s41598-021-88761-z
Schwartz MD, Hurst CG, Kirk MA, Reedy SJ, Braue EH. Reactive Skin Decontamination Lotion (RSDL) for the Decontamination of Chemical Warfare Agent (CWA) Dermal Exposure. Curr Pharm Biotechnol. 2012 Aug 1;13(10):1971-9. [PubMed Citation]
C. Link to non-clinical (e.g. animal) studies
Adult animal studies
-
Acute Toxicity
RSDL. Rabbit LD50 Dermal is greater than 950 mg/kg.
RSDL® (Reactive Skin Decontamination Lotion Kit). Safety Data Sheet 2017. https://www.qckslvr.com/Images/quicksilveranalytics/sds/_T__RSDL_-_Emergent_PN_10051.pdf(This 2017 Safety Data Sheet is noted but not linked to in Emergent BioSolutions Inc. RSDL Consumer website: https://rsdlready.com//p> -
Germ Cell Mutagencity.
-
RSDL. Bacterial Mutagenicity Assay (Salmonella typhimurium reverse mutation assay). Negative mutagenic response.
And
-
RSDL. In vivo Mouse Bone Marrow. Negative (non-genotoxic).
RSDL® (Reactive Skin Decontamination Lotion Kit). Safety Data Sheet 2017. https://www.qckslvr.com/Images/quicksilveranalytics/sds/_T__RSDL_-_Emergent_PN_10051.pdf(This 2017 Safety Data Sheet is noted but not linked to in Emergent BioSolutions Inc. RSDL Consumer website: https://rsdlready.com/
-
-
Reproductive Toxicity
RSDL. One generation reproduction study in rats. Maximum dose of 8 ml/kg/day dermally for 8.5 weeks in males and 2 weeks in females. No adverse reproductive effects.
RSDL. In vivo Mouse Bone Marrow. Negative (non-genotoxic).
RSDL® (Reactive Skin Decontamination Lotion Kit). Safety Data Sheet 2017. https://www.qckslvr.com/Images/quicksilveranalytics/sds/_T__RSDL_-_Emergent_PN_10051.pdf(This 2017 Safety Data Sheet is noted but not linked to in Emergent BioSolutions Inc. RSDL Consumer website: https://rsdlready.com/ -
Dermal Toxicity
[Pig Model.] Abstract. The organophosphate (OP) nerve agent VX is a weaponized chemical warfare agent that has also been used by terrorists against civilians. This contact poison produces characteristic signs of OP poisoning, including miosis, salivation, mastication, dysrhythmias and respiratory distress prior to death. Although successful treatment of OP poisoning can be obtained through decontamination and/or oxime reactivation of agent-inhibited cholinesterase, medical countermeasures that increase the therapeutic window for these measures would be of benefit. An anaesthetized swine model was utilized to examine the effects of lethal VX exposure to the skin, followed by cooling the exposure site prior to decontamination or treatment. The cooling was simply accomplished by using crushed ice in grip-seal plastic bags applied to the exposure sites. Cooling of skin exposed to lethal doses of VX significantly increased the window of opportunity for successful decontamination using the Reactive Skin Decontaminant Lotion® (RSDL®) or treatment with the oxime antidotes HI-6 and 2PAM. Analyses of blood VX levels showed that cooling acted to slow or prevent the entry of VX into the bloodstream from the skin. If the exposure site is known, the simple and non-invasive application of cooling provides a safe means with which to dramatically increase the therapeutic window in which decontamination and/or antidote treatment against VX are life-saving.
Sawyer TW, Mikler J, Worek F, Reiter G, Thiermann H, Tenn C, Weatherby K, Bohnert S. The therapeutic use of localized cooling in the treatment of VX poisoning. Toxicol Lett. 2011 Jul 4;204(1):52-6. [PubMed Citation]
-
[Guinea Pig Model.] Abstract. Low volatile organophosphorous nerve agents such as VX, will most likely enter the body via the skin. The pharmacokinetics of drugs such as oximes, atropine and diazepam, are not aligned with the variable and persistent toxicokinetics of the agent. Repeated administration of these drugs showed to improve treatment efficacy compared to a single injection treatment. Because of the effectiveness of continuous treatment, it was investigated to what extent a subchronic pretreatment with carbamate (pyridostigmine or physostigmine combined with either procyclidine or scopolamine) would protect against percutaneous VX exposure. Inclusion of scopolamine in the pretreatment prevented seizures in all animals, but none of the pretreatments affected survival time or the onset time of cholinergic signs. These results indicate that percutaneous poisoning with VX requires additional conventional treatment in addition to the current pretreatment regimen. Decontamination of VX-exposed skin is one of the most important countermeasures to mitigate the effects of the exposure. To evaluate the window of opportunity for decontamination, the fielded skin decontaminant Reactive Skin Decontaminant Lotion (RSDL) was tested at different times in hairless guinea pigs percutaneously challenged with 4x LD50 VX in IPA. The results showed that RSDL decontamination at 15 min after exposure could not prevent progressive blood cholinesterase inhibition and therefore would still require additional treatment. A similar decontamination regimen with RSDL at 90 min showed that it still might effectively increase the time window of opportunity for treatment. In conclusion, the delay in absorption presents a window of opportunity for decontamination and treatment. The continuous release of VX from the skin presents a significant challenge for efficacious therapy, which should ideally consist of thorough decontamination and continuous treatment.
Joosen MJ, van der Schans MJ, Kuijpers WC, van Helden HP, Noort D. Timing of decontamination and treatment in case of percutaneous VX poisoning: a mini review.Chem Biol Interact. 2013 Mar 25;203(1):149-53. [PubMed Citation]
[Pig Model.] “Abstract. Skin decontamination following exposure to chemical agents is a most important component of the individual defense doctrine, removing the agent, ceasing its penetration and preventing secondary contamination of the first responders. The goal of the current study was to compare the efficacy of Reactive Skin Decontaminant Lotion (RSDL) and Fuller's Earth (FE) following exposure to sulfur mustard (SM) and VX, aiming to find the optimal procedure for mass casualty decontamination protocol. Decontamination efficacy was evaluated in pigs by measurement of lesion area and erythema (SM) and cholinesterase inhibition and clinical symptoms (VX). FE and RSDL were highly effective against both agents. Following SM exposure, the two decontaminants demonstrated a significant decrease in lesions' size together with the decrease in exposure duration. Likewise, skin decontamination following exposure to VX with either FE or RSDL resulted in reduction in clinical symptoms and prevention of death. Decontamination was worthwhile even if postponed, up to 30 min (SM) and 2 h (VX). In conclusion, both decontamination products were efficient in ameliorating the toxic effects even though in a different mechanism. Finally, for mass casualty scenario, FE is preferred as a universal decontaminant, considering its safety, ease of use and longer shelf life.”
Also:
“The aim of the current study was to compare the efficacy of decontamination of FE with that of RSDL following dermal exposure to SM and VX and to recommend a universal protocol for military and civilian mass casualty scenarios. The work was performed in the pig model that was previously demonstrated as the preferred model for human skin [[16], [17], [18], [19]].”
and
“Removal of the CWA from the skin soon after exposure may reduce significantly the extent of skin damage following exposure to SM and the mortality rate after exposure to VX as well as enhancing the efficiency of present therapy.”Dachir S, Cohen M, Buch H, Kadar T. Skin decontamination efficacy of sulfur mustard and VX in the pig model: A comparison between Fuller's earth and RSDL. Chem Biol Interact. 2021 Feb 25;336:109393.
https://pubmed.ncbi.nlm.nih.gov/33508307/[PubMed Citation][Pig Ear Skin Samples.] Abstract. Organophosphorus compounds (OP), which mainly penetrate via the percutaneous pathway, represent a threat for both military and civilians. Body surface decontamination is vital to prevent victims poisoning. The development of a cost-effective formulation, which could be efficient and easy to handle in case of mass contamination, is therefore crucial. Metal oxides nanoparticles, due their large surface areas and the large amount of highly reactive sites, present high reactivity towards OP. First, this study aimed at evaluating the reaction of CeO2 nanoparticles, synthetized by microwave path and calcined at 500 or 600 °C, with Paraoxon (POX) in aqueous solution. Results showed that both nanoparticles degraded 60%-70% of POX. CeO2 calcined at 500 °C, owing to its larger specific area, was the most effective. Moreover, the degradation was significantly increased under Ultra-Violet irradiation (initial degradation rate doubled). Then, skin decontamination was studied in vitro using the Franz cell method with pig-ear skin samples. CeO2 powder and an aqueous suspension of CeO2 (CeO2-W) were applied 1 h after POX exposure. The efficiency of decontamination, including removal and/or degradation of POX, was compared to Fuller's earth (FE) and RSDL lotion which are, currently, the most efficient systems for skin decontamination. CeO2-W and RSDL were the most efficient to remove POX from the skin surface and decrease skin absorption by 6.4 compared to the control not decontaminated. FE reduced significantly (twice) the absorbed fraction of POX, contrarily to CeO2 powder. Considering only the degradation rate of POX, the products ranged in the order CeO2 > RSDL > CeO2-W > FE (no degradation). This study showed that CeO2 nanoparticles are a promising material for skin decontamination of OP if formulated as a dispersion able to remove POX like CeO2-W and to degrade it as CeO2 powder.
Salerno A, Devers T, Bolzinger MA, Pelletier J, Josse D, Briançon S. In vitro skin decontamination of the organophosphorus pesticide Paraoxon with nanometric cerium oxide CeO2.[PubMed Citation] Chem Biol Interact. 2017; 267:57-66. [PubMed Citation][Guinea Pig Model.] Abstract. In this study, we assessed the efficacy of the Reactive Skin Decontamination Lotion (RSDL®) Kit against parathion and aldicarb pesticide dermal exposure in a guinea pig model. The pesticides inhibit acetylcholinesterase (AChE) leading to signs and symptoms of hyperactivity of organs due to accumulation of acetylcholine. The RSDL Kit has been shown to physically remove and chemically degrade chemical warfare agents. Degradation occurs from a nucleophilic substitution reaction between an active ingredient in the RSDL lotion, potassium 2,3-butanedione monoximate (KBDO), with susceptible sites in these compounds. In the present study, guinea pigs dermally exposed to parathion and aldicarb were decontaminated with RSDL to mitigate the toxic effects of the pesticides. It is observed that animals exposed to 749 mg/kg of parathion (n = 3) died within 24 h without RSDL decontamination; however, RSDL-treated animals (n = 3) showed only mild signs of neurotoxicity. The RSDL-treated animals had an AChE inhibition of 0-58% while the untreated animals had up to 86% inhibition. Similarly, RSDL has been demonstrated to prevent aldicarb neurotoxicity effects. The percent inhibition of AChE activity during the 24 h post challenge of 9 mg aldicarb/kg of animal weight ranged from 25% to 61% with severe signs of intoxication while only up to 5% with mild or no signs of intoxication in the case of RSDL-decontaminated animals. Generally, it has been shown that the toxic effects of the organophosphate and carbamate pesticides can be prevented via decontamination using the RSDL Kit.
Fentabil M, Gebremedhin M, Barry J, Mikler J, Cochrane L. In vivo efficacy of the Reactive Skin Decontamination Lotion (RSDL®) kit against organophosphate and carbamate pesticides. Chem Biol Interact. 2020 Feb 25;318:108980.
https://pubmed.ncbi.nlm.nih.gov/32044340/[PubMed Citation][Pig Model.] Abstract. Research in skin decontamination and therapy of chemical warfare agents has been a difficult problem due to the simultaneous requirement of rapid action and nonaggressive behaviour. The aim of this study was to compare the performance of two decontaminating systems: the Canadian Reactive Skin Decontaminant Lotion (RSDL) and the Fuller's Earth (FE). The experiment was conducted with domestic swine, as a good model for extrapolation to human skin. RSDL and FE were tested against sulphur mustard (SM), a powerful vesicant, and VX, a potent and persistent cholinesterase inhibitor. When used 5 min after contamination, the results clearly showed that both systems were active against SM (10.1 mg/cm2) and VX (0.06 mg/cm2). The potency of the RSDL/sponge was statistically better than FE against skin injury induced by SM, observed 3 days post-exposure. RSDL was rather more efficient than FE in reducing the formation of perinuclear vacuoles and inflammation processes in the epidermis and dermis. Against a severe inhibition (67%) of plasmatic cholinesterases induced by VX poisoning, the potencies of the RSDL/sponge and FE were similar. Both systems completely prevented cholinesterase inhibition, which indirectly indicates a prevention of toxic absorption through the skin.
Taysse L, Daulon S, Delamanche S, Bellier B and Breton P.Skin decontamination of mustards and organophosphates: comparative efficiency of RSDL and Fuller's earth in domestic swine. Hum Exp Toxicol. 2007 Feb;26(2):135-41. [PubMed Citation]
[Pig Model.] Abstract. The chemical weapon nerve agent known as Russian VX (VR) is a potent organophosphorus (OP) compound that is much less studied than its VX analogue with respect to toxicity, as well as to the effectiveness of several known countermeasures against it. An anaesthetized domestic swine model was utilized to assess several approaches in mitigating its toxicity, including the utility of cooling VR treated skin to increase the therapeutic window for treatment. The 6 h LD50 for VR topically applied on the ear was determined. Treatment of VR exposed animals (5x LD50) with pralidoxime (2PAM) very poorly regenerated inhibited blood cholinesterase activity, but was partially effective in preventing signs of OP poisoning and increasing survival. In contrast, treatment with the Hagedorn oxime HI-6 reactivated cholinesterase, eliminated all signs of poisoning and prevented death. Decontamination with the Reactive Skin Decontaminant Lotion (RSDL) 15 min after VR exposure was completely effective in preventing death. Cooling of the VR exposure sites for 2 or 6 h prevented signs of OP poisoning and death during the cooling period. However, these animals died very quickly after the cessation of cooling, unless they were treated with oxime or decontaminated with RSDL. Blood analyses showed that cooling of agent exposure sites delayed the entry of VR into the bloodstream. Medical treatment with HI-6 and to a lesser extent 2PAM, or decontamination with RSDL are effective in protecting against the toxic effects of cutaneous exposure to VR. Immobilizing this agent (and related compounds) within the dermal reservoir by cooling the exposure sites, dramatically increases the therapeutic window in which these medical countermeasures are effective.
Mikler J, Tenn C, Worek F, Reiter G, Thiermann H, Garrett M, Bohnert S, Sawyer TW.Immobilization of Russian VX skin depots by localized cooling: Implications for decontamination and medical countermeasures . Toxicol Lett. 2011 Sep 25;206(1):47-53. [PubMed Citation]
[Pig Model.] Abstract. An anesthetized domestic swine model was used to compare the efficacy and cross-contamination potential of selected skin decontaminant products and regimens against the chemical warfare agent, VX. Animals topically exposed to 2x, 3x or 5x LD50 VX showed typical signs of organophosphate nerve agent poisoning, including miosis, salivation, mastication, dysrhythmias, and respiratory distress prior to death. Animals were exposed to 5x LD50 VX and then decontaminated 45 min later with the reactive skin decontamination lotion (RSDL®), Fuller's earth (FE), 0.5% hypochlorite, or soapy water. Survival was 100% when the reactive skin decontamination lotion or FE was utilized, although 50% of Fuller's earth decontaminated animals exhibited serious signs of VX poisoning. Decontamination of VX-treated animals with 0.5% hypochlorite was less effective but also increased survival. Soapy water was ineffective in preventing lethality. Blood cholinesterase levels were not predictive of clinical outcome in decontaminated animals. The potential of "decontaminated" VX in open wounds to cause poisoning was assessed by vigorously mixing 5x LD50 VX with the test decontaminants for 5 min and then placing the mixture onto a full-thickness skin wound. Soapy water was ineffective in preventing lethality. Although treatment with dry Fuller's earth prevented death and all signs of organophosphate poisoning, a significant proportion of treated animals decontaminated with Fuller's earth in aqueous suspension exhibited serious signs of organophosphate poisoning, suggesting that live agent may be desorbed from Fuller's earth when it is exposed to a liquid environment. Animals treated with reactive skin decontamination lotion or 0.5% hypochlorite-VX mixtures showed no signs of organophosphate poisoning during the 6-h test period.
Bjarnason S, Mikler J, Hill I, Tenn C, Garrett M, Caddy N and Sawyer TW.Comparison of selected skin decontaminant products and regimens against VX in domestic swine . Hum Exp Toxicol. 2008 Mar;27(3):253-61. [PubMed Citation]
[Mouse Model.] Abstract. Using the hairless mouse screening model presented in the companion paper the aim of this study was to assess two skin decontaminating systems: Fuller's earth (FE) and Reactive Skin Decontamination Lotion (RSDL)against two extremely toxic chemical warfare agents that represent a special percutaneous hazard, sulphur mustard (SM) and O-ethyl-S-(2[di-isopropylamino]ethyl)methyl-phosphonothioate (VX). Five minutes after being exposed on the back to either 2 mL of neat sulphur mustard or 50 mg/kg of diluted VX, mice were decontaminated. Both systems were able to reduce blisters 3 days after SM exposure. However, RSDL was found to be more efficient than FE in reducing the necrosis of the epidermis and erosion. In the case of VX exposure, RSDL, whatever the ratio of decontaminant to toxicant used (RSDL 10, 20, 50), was not able to sufficiently prevent the inhibition of plasma cholinesterases taken as a surrogate marker of exposure and toxicity. Only FE reduced significantly the ChE inhibition. Some of these observations are different from our previous results obtained in domestic swine and these changes are thus discussed in the perspective of using SKH-1 hairless mice for the initial in vivo screening of decontaminants.
Taysse L, Dorandeu F, Daulon S, Foquin A, Perrier N, Lallement G and Breton P.Cutaneous challenge with chemical warfare agents in the SKH-1 hairless mouse (II): Effects of some currently used skin decontaminants (RSDL and Fuller's earth) against liquid sulphur mustard and VX exposure . Hum Exp Toxicol. 2011 Jun;30(6):491-8. [PubMed Citation]
[Guinea Pig Model.] Abstract. There have been numerous studies of the central nervous system (CNS) involvement in organophosphate (OP) poisoning showing status epilepticus and/or 'electrographic seizures'. Brain damage has been demonstrated as 'neuronal necrosis' primarily in the cortex, thalamus and hippocampus. To the authors' knowledge there have been no reports of partial/total paralysis following close upon OP exposure although delayed paralysis has been reported. This report summarizes the immediate, OP induced paralytic events recorded in guinea pigs during development of the Canadian reactive skin decontaminant lotion (RSDL®). As part of the development work, supra-lethal cutaneous doses of OP were applied to large numbers of guinea pigs followed by decontamination with the RSDL® or predecessor lotions and solvents. Soman (pinacolyl methylphosphonofluoridate; GD) challenges were applied to 1277 animals and S-(2-diisopropyl-aminoethyl) methylphosphorothiolate (VX) challenges to 108. The classic sequence of clinical signs - ptyalism, tremors, fasciculations, convulsions, apnea and flaccid paralysis before death - was seen in the 658 animals that died and in many of the survivors. Eighty-four of 688 survivors of GD and 4 of 39 survivors of VX showed random paralysis of various distal regions following recovery from an insult which produced convulsions and/or flaccid paralysis. Because the experiments were designed to assess the decontamination procedures, there were no apparent relationships between the amounts of OP applied and the sequellae recorded. The observations of paralysis were also incidental to the prime focus of the experiments. Because of this, only ten animals paralysed following GD exposure were examined for histological effects. The pathologist diagnosed 'encephalomalacia' and 'focal necrotic lesions' in the cerebral cortex and 'focal necrotic lesions' in one spinal cord. Of the 84 guinea pigs paralysed after GD challenge, one was not decontaminated and the decontaminants used on the remainder were sufficiently varied that there appeared to be no relationship between the type of decontaminant and the resulting paralysis.
Bide RW, Schofield L and Risk DJ.Immediate post-dosing paralysis following severe Soman and VX toxicosis in guinea pigs. J Appl Toxicol. 2005 Sep-Oct;25(5):410-7. [PubMed Citation]
[Guinea Pig Model.] Abstract. Objective: This report, the second in a series of five, directly compares the efficacy of Reactive Skin Decontamination Lotion (RSDL), the M291 Skin Decontamination Kit (SDK), 0.5% bleach (sodium or calcium hypochlorite solution), 1% soapy water, and Skin Exposure Reduction Paste Against Chemical Warfare Agents (SERPACWA) in the haired guinea pig model following exposure to soman (GD). Methods: In all experiments, guinea pigs were close-clipped and given anesthesia. In the decontamination experiments, the animals were challenged with GD and decontaminated after a 2-minute delay for the standard procedure or at longer times for the delayed-decontamination experiments. Positive control animals were challenged with GD in the same manner as the treated animals, except that they received no treatment. All animals were observed during the first 4 hours and again at 24 hours after exposure for signs of toxicity and death. The protective ratio (PR, defined as the median lethal dose [LD50] of the treatment group divided by the LD50 of the untreated positive control animals) was calculated from the derived probit dose-response curves established for each treatment group and nontreated control animals. SERPACWA was applied as a thin coating (0.1 mm thick), allowed to dry for 15 minutes, and challenged with GD. After a 2-hour challenge, any remaining GD was blotted off the animal, but no additional decontamination was done. Significance in this report is defined as p <.05. Neat (undiluted) GD was used to challenge all animals in these studies. Results: In the standard 2-minute GD decontamination experiments, the calculated PRs for RSDL, 0.5% bleach, 1% soapy water, and M291 SDK were 14, 2.7, 2.2, and 2.6, respectively. RSDL was by far the most effective decontamination product tested and significantly better than any of the other products. Bleach, soapy water, and the M291 SDK provided equivalent and modest protection. Since only RSDL provided at least good protection (PR > 5), it was the only decontamination product evaluated for delayed decontamination. In the GD delayed decontamination experiments, the calculated LT50 (the delayed-decontamination time at which 50% of the animals die in the test population following a 5-LD50 challenge) value for RSDL was only 4.0 minutes.
Conclusions: Several conclusions can be drawn from this study: 1) Reactive Skin Decontamination Lotion provided superior protection against GD compared with the other products tested; 2) The 0.5% bleach solution, the 1% soapy water solution, and the M291 SDK were less effective than RSDL, but still provided modest (2 < PR < 5) protection against GD; 3) Reactive Skin Decontamination Lotion, the best product tested, did not provide significant protection against GD when decontamination was delayed for more than 3 minutes; 4) Skin Exposure Reduction Paste Against Chemical Warfare Agents provided significant, but modest, protection against GD; 5) There was good correlation between using the rabbit model and the guinea pig model for decontamination efficacy evaluations; and 6) Soman (GD) is an agent of real concern because it is very difficult to decontaminate and the effects of exposure are difficult to treat.
Braue EH Jr, Smith KH, Doxzon BF, Lumpkin HL, Clarkson ED. Efficacy studies of Reactive Skin Decontamination Lotion, M291 Skin Decontamination Kit, 0.5% bleach, 1% soapy water, and Skin Exposure Reduction Paste Against Chemical Warfare Agents, Part 2: Guinea pigs challenged with soman. Cutan Ocul Toxicol. 2011 Mar;30(1):29-37. [PubMed Citation]
[Rat Model.] Reactive skin decontamination lotion (RSDL) is a proposed replacement for the existing skin and equipment decontamination kit. Because RSDL may need to be used to decontaminate wounded personnel, we conducted an assessment of the effect of this agent on wound healing. A skin incision model using male Sprague Dawley rats (n = 19 rats/group) was used. A 7.0-cm incision was made through the skin, and RSDL was (experimental group) or was not (control group) applied to the open wound; the wound edges were then approximated with sutures. Seven days later, animals were euthanized and wound samples were taken. Healing was assessed by measuring mechanical strength, collagen content, and histological appearance. RSDL-treated wounds had 23% lower tensile strength (p < 0.05) and 11% lower collagen content (p < 0.05) than did the untreated control wounds. Histological assessments did not differ significantly between groups. The results of this investigation demonstrate that the application of RSDL directly to an open wound impairs wound strength and decreases collagen content in the early phases of wound healing. This may have clinical implications for the treatment and outcomes of chemical casualty combat trauma.
Walters TJ, Kauvar DS, Reeder J, Baer DG. Effect of Reactive Skin Decontamination Lotion on Skin Wound Healing in Laboratory Rats. Mil Med. 2007 Mar;172(3):318-21. [PubMed Citation]
[Guinea Pig Model.]This report, first in a series of five, directly compares the efficacy of 4 decontamination products and Skin Exposure Reduction Paste Against Chemical Warfare Agents (SERPACWA) in the haired guinea pig model following exposure to VX. In all experiments, guinea pigs were close-clipped and given anesthesia. In the decontamination experiments, the animals were challenged with VX and decontaminated after a 2-minute delay for the standard procedure or at longer times for the delayed-decontamination experiments. Skin Exposure Reduction Paste Against Chemical Warfare Agents was applied as a thin coating (0.1 mm thick), allowed to dry for 15 minutes, and challenged with VX. After a 2-hour challenge, any remaining VX was blotted off the animal, but no additional decontamination was done. Positive control animals were challenged with VX in the same manner as the treated animals, except that they received no treatment. In addition, the positive control animals were always challenged with 5% VX in isopropyl alcohol (IPA) solution, whereas the treatment animals received either neat (undiluted) VX or 5% VX in IPA solution. All animals were observed during the first 4 hours and again at 24 hours after exposure for signs of toxicity and death. The protective ratio (PR, defined as the median lethal dose [LD(50)] of the treatment group divided by the LD(50) of the untreated positive control animals) was calculated from the probit dose-response curves established for each treatment group and nontreated control animals. Significance in this report was defined as p < .05. In the standard 2-minute neat VX decontamination experiments, the calculated PRs for Reactive Skin Decontamination Lotion (RSDL), 0.5% bleach, 1% soapy water, and the M291 Skin Decontamination Kit (SDK) were 66, 17, 16, and 1.1, respectively. Reactive Skin Decontamination Lotion was by far the most effective decontamination product tested and was significantly better than any of the other products. Bleach and soapy water provided equivalent and good (PR > 5) protection. They were both significantly better than the M291 SDK. The M291 SDK did not provide significant protection compared with positive controls. In the neat VX delayed-decontamination experiments, the calculated LT(50) (the delayed-decontamination time at which 50% of the animals died in the test population following a 5-LD(50) challenge) values for RSDL, 0.5% bleach, and 1% soapy water were 31, 48, and 26 minutes, respectively. The results showed that SERPACWA provided significant, but modest (PR < 5), protection against neat VX, with a PR of 2.1. Several conclusions can be drawn from this study: 1) RSDL provided superior protection against VX compared with the other products tested; 2) 0.5% bleach and 1% soapy water were less effective than RSDL, but still provided good protection against VX; 3) the M291 SDK was the least effective decontamination product and did not provide significant protection against VX; 4) the agent was observed to streak when using the M291 SDK, and efficacy may improve if the agent is first blotted, followed by wiping with a new or clean part of the M291 SDK pad; 5) RSDL, 0.5% bleach, and 1% soapy water provided significant protection against a 5-LD(50) challenge of VX, even when decontamination was delayed for up to about 30 minutes; and 6) SERPACWA provided significant, but modest, protection against VX.
Braue EH Jr, Smith KH, Doxzon BF, Lumpkin HL, Clarkson ED. Efficacy studies of Reactive Skin Decontamination Lotion, M291 Skin Decontamination Kit, 0.5% bleach, 1% soapy water, and Skin Exposure Reduction Paste Against Chemical Warfare Agents, part 1: guinea pigs challenged with VX. Cutan Ocul Toxicol. 2011 Mar;30(1):15-28. [PubMed Citation]
[Pig Model.] Abstract. Reactive Skin Decontamination Lotion (RSDL®) is an FDA-approved skin decontamination kit carried by service members for removal and neutralisation of vesicants and nerve agents. The RSDL kit, comprised of a lotion-impregnated sponge, was shown to be the superior medical decontamination device for chemical warfare agent (CWA) exposure on intact skin. In the event of a chemical exposure situation (i.e. terrorism, battlefield) physical injuries are probable, and preservation of life will outweigh the risk associated with application of RSDL to compromised skin. The purpose of this study was to quantify the rate and quality of wound healing in epidermal skin wounds treated with RSDL in a porcine model. Degree of wound healing was assessed using bioengineering methods to include ballistometry, colorimetry, evaporimetry, and high-frequency ultrasonography. Clinical observation, histopathology and immunohistochemistry were also utilised. All pigs received four bilateral superficial abdominal wounds via a pneumatic dermatome on their ventral abdomen, then were treated with the following dressings over a seven-day period: RSDL sponge, petroleum based Xeroform® gauze, 3 M™ Tegaderm™ Film, and 3 M™ Tegaderm™ Foam. Two additional non-wounded sites on the flank were used as controls. Two groups of pigs were then evaluated for a 21- or 56-day time period, representing short- and long-term wound-healing progression. Our findings indicated RSDL had a negative impact on wound-healing progression at both 21 and 56 days post-injury. Wounds receiving RSDL demonstrated a decreased skin elasticity, significant transepidermal water loss, and altered skin colouration and thickness. In addition, the rate of wound healing was delayed, and return to a functional skin barrier was altered when compared to non-RSDL-treated wounds. In conclusion, wound management care and clinical therapeutic intervention plans should be established to account for a prolonged duration of healing in patients with RSDL-contaminated wounds.
Connolly JM, Stevenson RS, Railer RF, Clark OE, Whitten KA, Lee-Stubbs RB, Anderson DR. Impairment of wound healing by reactive skin decontamination lotion (RSDL®) in a Göttingen minipig® model. Cutan Ocul Toxicol. 2020 Jun;39(2):143-157.
https://pubmed.ncbi.nlm.nih.gov/32321319/ [ [PubMed Citation]Pregnant animal studies
-
RSDL should be used during pregnancy only when necessary; one of the ingredients; 2,3 butanedione monoxime (DAM) has been shown to cross the placental barrier in animal studies. (1) Animal reproduction studies have shown RSDL to be non-toxic for all of the reproductive parameters examined, including the neonates – RSDL is not teratogenic, not spermicidal, and not embriocidal. (2) No human studies on pregnant women have been conducted.
RSDL® (Reactive Skin Decontamination Lotion Kit). Safety Data Sheet 2017.
https://www.qckslvr.com/Images/quicksilveranalytics/sds/_T__RSDL_-_Emergent_PN_10051.pdf(This 2017 Safety Data Sheet is noted but not linked to in Emergent BioSolutions Inc. RSDL Consumer website: https://rsdlready.com/Reproductive Toxicity RSDL. One generation reproduction study in rats. Maximum dose of 8 ml/kg/day dermally for 8.5 weeks in males and 2 weeks in females. No adverse reproductive effects.
RSDL® (Reactive Skin Decontamination Lotion Kit). Safety Data Sheet 2017.
https://www.qckslvr.com/Images/quicksilveranalytics/sds/_T__RSDL_-_Emergent_PN_10051.pdf(This 2017 Safety Data Sheet is noted but not linked to in Emergent BioSolutions Inc. RSDL Consumer website: https://rsdlready.com/
Other non-clinical studies
Comparative human and animal in vitro studies-
The chemical warfare agents such as VX represent a threat for both military and civilians, which involves an immediate need of effective decontamination systems. Since human scalp is usually unprotected compared to other body regions covered with clothes, it could be a preferential site of exposure in case of terrorist acts. The purpose of this study was to determine if skin decontamination could be efficient when performed more than 1h after exposure. In addition, the impact of hairs in skin contamination was investigated. By using in vitro skin models, we demonstrated that about 75% of the applied quantity of VX was recovered on the skin surface 2h after skin exposition, which means that it is worth decontaminating even if contamination occurred 2h before. The stratum corneum reservoir for VX was quickly established and persistent. In addition, the presence of hairs modified the percutaneous penetration of the nerve agent by binding of VX to hairs. Hair shaft has thus to be taken into account in the decontamination process. Reactive Skin Decontamination Lotion (RSDL) and Fuller's Earth (FE) were active in the skin decontamination 45min post-exposure, but RSDL was more efficient in reducing the amount of VX either in the skin or in the hair.
Rolland P, Bolzinger MA, Cruz C, Josse D, Briançon S. Hairy skin exposure to VX in vitro: effectiveness of delayed decontamination. Toxicol In Vitro. 2013 Feb;27(1):358-66. [PubMed Citation]
-
[In vitro, Utilizing a Diffusion Cell and Dermatomed Human Skin.] The decontamination efficacy of four commercially available skin decontamination products following exposure to the nerve agent VX was evaluated in vitro utilizing a diffusion cell and dermatomed human skin. The products included were Reactive Skin Decontamination Lotion (RSDL), the Swedish decontamination powder 104 (PS104), the absorbent Fuller's Earth and the aqueous solution alldecontMED. In addition, various decontamination procedures were assessed to further investigate important mechanisms involved in the specific products, e.g. decontamination removal from skin, physical removal by sponge swabbing and activation of degradation mechanisms. The efficacy of each decontamination product was evaluated 5 or 30 min after dermal application of VX (neat or diluted to 20% in water). The RSDL-lotion was superior in reducing the penetration of VX through human skin, both when exposed as neat agent and when diluted to 20% in water. Swabbing with the RSDL-sponge during 2 min revealed decreased efficacy compared to applying the RSDL-lotion directly on the skin for 30 min. Decontamination with Fuller's Earth and alldecontMED significantly reduced the penetration of neat concentration of VX through human skin. PS104-powder was insufficient for decontamination of VX at both time-points, independently of the skin contact time of PS104. The PS104-slurry (a mixture of PS104-powder and water), slightly improved the decontamination efficacy. Comparing the time-points for initiated decontamination revealed less penetrated VX for RSDL and Fuller's Earth when decontamination was initiated after 5 min compared to 30 min post-exposure, while alldecontMED displayed similar efficacy at both time-points. Decontamination by washing with water only resulted in a significant reduction of penetrated VX when washing was performed 5 min after exposure, but not when decontamination was delayed to 30 min post-exposure of neat VX. In conclusion, early initiated decontamination with the RSDL-lotion, containing both absorption and degrading properties, allowed to act on skin for 30 min was superior in preventing VX from penetrating human skin. Adding water during decontamination resulted in increased penetration of neat VX, however, water in the decontaminant removal process did not influence the decontamination efficacy. From our study on commercially available decontaminants, it is recommended that future product developments should include both strong absorbents and efficient nerve agent degrading components.
Thors L, Koch M, Wigenstam E, Koch B, Hägglund L, Bucht A.Comparison of skin decontamination efficacy of commercial decontamination products following exposure to VX on human skin .Chem Biol Interact. 2017 Aug 1;273:82-89. [PubMed Citation]
-
[In vitro, Using Human Skin.] Abstract. Dermal exposure to low volatile organophosphorus compounds (OPC) may lead to penetration through the skin and uptake in the blood circulation. Skin decontamination of toxic OPCs, such as pesticides and chemical warfare nerve agents, might therefore be crucial for mitigating the systemic toxicity following dermal exposure. Reactive skin decontamination lotion (RSDL) has been shown to reduce toxic effects in animals dermally exposed to the nerve agent VX. In the present study, an in vitro flow-through diffusion cell was utilized to evaluate the efficacy of RSDL for decontamination of VX exposed to human epidermis. In particular, the impact of timing in the initiation of decontamination and agent dilution in water was studied. The impact of the lipophilic properties of VX in the RSDL decontamination was additionally addressed by comparing chemical degradation in RSDL and decontamination efficacy between the VX and the hydrophilic OPC triethyl phosphonoacetate (TEPA). The epidermal membrane was exposed to 20, 75 or 90% OPC diluted in deionized water and the decontamination was initiated 5, 10, 30, 60 or 120 min post-exposure.
Early decontamination of VX with RSDL, initiated 5–10 min after skin exposure, was very effective. Delayed decontamination initiated 30–60 min post-exposure was less effective but still the amount of penetrated agent was significantly reduced, while further delayed start of decontamination to 120 min resulted in very low efficacy. Comparing RSDL decontamination of VX with that of TEPA showed that the decontamination efficacy at high agent concentrations was higher for VX. The degradation mechanism of VX and TEPA during decontamination was dissected by 31P NMR spectroscopy of the OPCs following reactions with RSDL and its three nucleophile components. The degradation rate was clearly associated with the high pH of the specific solution investigated; i.e. increased pH resulted in a more rapid degradation. In addition, the solubility of the OPC in RSDL also influenced the degradation rate since the degradation of VX was significantly faster when the NMR analysis was performed in the organic solvent acetonitrile compared to water.
In conclusion, we have applied the in vitro flow-through diffusion cell for evaluation of skin decontamination procedures of human epidermis exposed to OPCs. It was demonstrated that early decontamination is crucial for efficient mitigation of epidermal penetration of VX and that almost complete removal of the nerve agent from the skin surface is possible. Our data also indicate that the pH of RSDL together with the solubility of OPC in RSDL are of primary importance for the decontamination efficacy.
Highlights
- Early initiated decontamination of VX with RSDL on human skin was efficient.
- RSDL-effectiveness was greatly reduced with extended OPC-exposure times.
- RSDL-efficacy was dependent on the chemical properties and concentrations of the OPCs.
- High pH and OPC-solubility were crucial for agent degradation by RSDL
Thors L, Lindberg S, Johansson S, Koch B, Koch M, Hägglund L, Bucht A. RSDL decontamination of human skin contaminated with the nerve agent VX. Toxicol Lett. 2017 Mar 5;269:47-54. https://pubmed.ncbi.nlm.nih.gov/28179194/ [PubMed Citation]
-
[In Vitro, Using Human Skin.] Abstract. This study compared the efficiency for in vitro human skin decontamination using DDGel and RSDL when applied at different timepoints (5 min and 90 min). Experiments were performed using in vitro human skin models, in which skin was mounted onto Flow-Through diffusion cells. The mass of 14-C DIMP removed from skin surface after decontamination was quantitated by measuring radioactivity with a liquid scintillation spectrometer. Both decontaminants removed more than 90% recovery dose of DIMP from skin compared to control group (p < 0.05). DDGel skin decontamination reduced more toxicant amount when compared to RSDL. DDGel showed slight higher decontamination ability of DIMP than RSDL and efficiently removed chemicals from the skin surface, also reduced the amount of DIMP in receptor fluid. A similar decontamination regimen with RSDL and DDGel at 90 min showed that both were still might effectively increase the time window of opportunity for treatment. Thus, DDGel can offer a potential as a next generation skin decontamination platform technology for military and civilian applications.
Highlights
- Both DDGel and RSDL decontaminants removed more than 90% recovery dose of DIMP from skin surface.
- DDGel skin decontamination reduced more toxicant amount when compared to RSDL.
- Decontamination results at 90 min of DDGel and RSDL showed they might increase the time window of opportunity for treatment.
Cao Y, Hui X, Elmahdy A, Maibach H. In vitro human skin permeation and decontamination of diisopropyl methylphosphonate (DIMP) using Dermal Decontamination Gel (DDGel) and Reactive Skin Decontamination Lotion (RSDL) at different timepoints. Toxicol Lett. 2018 Dec 15;299:118-123. https://pubmed.ncbi.nlm.nih.gov/30282006/ xc [PubMed Citation]
4. Pharmacokinetic and toxicokinetics data
— including children-, pregnancy-, geriatric-, and obesity-related dataToxicokinetics.
Human Phase 1 PK Topical Application of RSDL. DAM is absorbed through human skin. Heat stress increased uptake. Washing to remove RSDL limited further uptake of DAM.
RSDL® (Reactive Skin Decontamination Lotion Kit). Safety Data Sheet 2017.
https://www.qckslvr.com/Images/quicksilveranalytics/sds/_T__RSDL_-_Emergent_PN_10051.pdf (This 2017 Safety Data Sheet is noted but not linked to in Emergent BioSolutions Inc. RSDL Consumer website: https://rsdlready.com/)-
Abstract. This study examined the degradation of organophosphate (OP) and carbamate pesticides using RSDL® (Reactive Skin Decontamination Lotion Kit) lotion. Degradation occurs from a nucleophilic substitution (SN) reaction between an ingredient in the RSDL lotion, potassium 2,3-butanedione monoximate (KBDO), with susceptible sites in the pesticides. Evaluation at several molar ratios of KBDO:test articles using liquid chromatography-mass spectrometry (LC-MS) techniques was performed. The OP test articles, parathion, paraoxon, parathion-methyl, paraoxon-methyl and chlorpyrifos were effectively degraded at molar ratios of four and above in less than 6min contact time. Malathion and malaoxon were similarly converted to inactive by-products at molar ratios as low as two in less than 4min. A minimum molar ratio of nine was found to be effective against the carbamate pesticide carbofuran. In the case of aldicarb, complete destruction was achieved at a molar ratio of fifteen and a reaction time of one hour. It is important to note that these studies are based on a direct liquid phase RSDL lotion reaction with the toxic chemicals without the added physical removal decontamination efficacy component provided by the sponge component of the RSDL kit. The RSDL kit is intended to be used to remove or neutralize chemical warfare agents (CWA) and T-2 toxin from the skin. In actual use, the majority of the CWA decontamination occurs through the combined action of the sponge in both removing the chemical from the skin, and in rapidly mixing the chemicals at a high molar ratio of KBDO:CWA within the pores of the sponge to enhance rapid neutralization of the chemical.
Fentabil M, Gebremedhin M, Purdon JG, Cochrane L, Goldman VS. Degradation of pesticides with RSDL® (reactive skin decontamination lotion kit) lotion: LC-MS investigation. Toxicol Lett. 2018 Sep 1;293:241-248. https://pubmed.ncbi.nlm.nih.gov/29128639/ [PubMed Citation]
-
Abstract. Skin decontamination in cold weather temperatures might be challenging due to the aggravating circumstances. However, no information is available on the efficacy of commonly used procedures in winter conditions. Therefore, the efficacy of the reactive skin decontamination lotion (RSDL) and soapy water decontamination following skin exposure to the nerve agent VX was evaluated at three ambient air temperatures (-5°C, -15°C and room temperature). Experiments were performed in vitro using human dermatomed skin. The ability of RSDL to degrade VX at the three different air temperatures was separately evaluated. The ambient air temperature in experiments without decontamination did not influence the penetration rate of VX through skin. RSDL decontamination was highly efficient in removing VX from skin when performed in all three ambient temperatures, despite the slower agent degradation rate of VX at the lower temperatures. Decontamination with soapy water at RT resulted in an increased skin penetration of VX compared with the control without decontamination; however, in colder temperatures the VX skin penetration was similar to the corresponding control without decontamination. At RT, dry removal prior to washing with soapy water did not improve decontamination of VX compared with washing solely with soapy water. This study demonstrated high efficacy of RSDL decontamination following skin exposure to VX also at cold temperatures. The previously reported 'wash-in' effect of soapy water on VX skin penetration was reduced at cold temperatures. Altogether, this study found a scientific basis to establish guidelines for skin decontamination of chemical casualties at cold weather temperatures.
Thors L, Wästerby P, Wigenstam E, Larsson A, Öberg L, Bucht A. Do cold weather temperatures affect the efficacy of skin decontamination? J Appl Toxicol. 2022 Jun;42(6):961-969. https://pubmed.ncbi.nlm.nih.gov/34850419/ [PubMed Citation]
-
Abstract. Chemical warfare agents are an actual threat and victims' decontamination is a main concern when mass exposure occurs. Skin decontamination with current protocols has been widely documented, as well as surface decontamination. However, considering hair ability to trap chemicals in vapour phase, we investigated hair decontamination after exposure to sulphur mustard simulants methyl salicylate and 2-chloroethyl ethyl sulphide. Four decontamination protocols were tested on hair, combining showering and emergency decontamination (use of Fuller's earth or Reactive Skin Decontamination Lotion RSDL®). Both simulants were recovered from hair after treatment, but contents were significantly reduced (42-85% content allowance). Showering alone was the least efficient protocol. Concerning 2-chloroethyl ethyl sulphide, protocols did not display significant differences in decontamination efficacy. For MeS, use of emergency decontaminants significantly increased showering efficacy (10-20% rise), underlining their usefulness before thorough decontamination. Our results highlighted the need to extensively decontaminate hair after chemical exposure. Residual amounts after decontamination are challenging, as their release from hair could lead to health issues.
Spiandore M, Piram A, Lacoste A, Prevost P, Maloni P, Torre F, Asia L, Josse D, Doumenq P. Efficacy of scalp hair decontamination following exposure to vapours of sulphur mustard simulants 2-chloroethyl ethyl sulphide and methyl salicylate. Chem Biol Interact. 2017 Apr 1;267:74-79. https://pubmed.ncbi.nlm.nih.gov/27492218/ [PubMed Citation]
-
Abstract. Early initiated decontamination is demonstrated to be crucial to avoid systemic effects of highly toxic and low volatile agents exposed on the skin. Skin decontamination can be performed by simple procedures, such as washing with soap and water, or by using advanced decontamination products containing absorption and agent degradation properties. Reactive Skin Decontamination Lotion (RSDL) has demonstrated high efficacy to remove nerve agents from the skin. However, contrary to the current operational recommendations, experimental studies have shown that prolonged skin contact time of RSDL is important for efficient decontamination of VX. In the present study, several RSDL-protocols were evaluated for the efficacy to remove neat VX from human skin in vitro. The decontamination efficacies of the RSDL-procedures were compared with the efficacy of the simple procedure of washing off the skin with soapy water. The RSDL-protocols containing repeated swabbing with the sponge and a 10 min skin contact time of RSDL-lotion demonstrated the greatest decontamination efficacy of all procedures evaluated. Repeating the protocol 2 h after the initial decontamination step resulted in a transient increased skin penetration of remaining intact agent on skin and was followed by rapidly declined agent penetration rate. Decontamination performed with soapy water significantly increased agent amounts penetrating skin, most likely caused by skin hydration and agent dilution. In conclusion, a slightly extended procedure for RSDL-decontamination showed improved efficacy and is therefore recommended for removal of nerve agents from the skin. In addition, it is of highest importance that skin decontamination of nerve agents should consist of procedures using low water content.
Thors L, Wigenstam E, Qvarnström J, Hägglund L, Bucht A. Improved skin decontamination efficacy for the nerve agent VX. Chem Biol Interact. 2020 Jul 1;325:109-135. https://pubmed.ncbi.nlm.nih.gov/32428449/ [PubMed Citation]
Pregnancy
-
RSDL should be used during pregnancy only when necessary; one of the ingredients; 2,3 butanedione monoxime (DAM) has been shown to cross the placental barrier in animal studies. (1) Animal reproduction studies have shown RSDL to be non-toxic for all of the reproductive parameters examined, including the neonates – RSDL is not teratogenic, not spermicidal, and not embriocidal. (2) No human studies on pregnant women have been conducted.
RSDL® (Reactive Skin Decontamination Lotion Kit). Safety Data Sheet 2017.
https://www.qckslvr.com/Images/quicksilveranalytics/sds/_T__RSDL_-_Emergent_PN_10051.pdf(This 2017 Safety Data Sheet is noted but not linked to in Emergent BioSolutions Inc. RSDL Consumer website: https://rsdlready.com/)
5. Current FDA/EUA approved indications and dosing
— including children-, pregnancy-, geriatric-, and obesity-related data, and Emergency Use Authorization (EUA)Adults (FDA)
-
Usage:
The full strength solution is applied on body surfaces after exposure to chemical warfare agents (RSDL should not be used before exposure since its effectiveness following prophylactic use has not been evaluated). Generally, one 21 mL packet is sufficient to decontaminate hands, neck, and face. The packaging and sponge should be discarded after single use. -
How Supplied:
The FDA has cleared the use of RSDL in 21 and 42 mL packets. Each ml of solution contains 1.25 M or 173 mg of Dekon 139 and 35-43 mg of DAM in an MPEG and water solvent.
(Emergent BioSolutions Inc. web site “for professional audiences in the U.S.”)
Pregnancy (FDA)
-
DAM has been shown to cross the placental barrier but teratogenicity studies have not been performed ; thus RSDL should only be used during pregnancy when absolutely necessary. RSDL has been shown to be non-mutagenic and inhalation and reproductive studies in rats have shown RSDL not to cause any ill effects
RSDL® (Reactive Skin Decontamination Lotion Kit). Safety Data Sheet 2017.
https://www.qckslvr.com/Images/quicksilveranalytics/sds/_T__RSDL_-_Emergent_PN_10051.pdf (This 2017 Safety Data Sheet is noted but not linked to in Emergent BioSolutions Inc. RSDL Consumer website: https://rsdlready.com/ )
Emergency Use Authorization (FDA/CDC)
-
No Emergency Use Authorization for RSDL has been issued from the Food and Drug Administration under section 564 of the Federal Food, Drug and Cosmetic Act (FD&C Act) (21 U.S.C. 360bbb-3), amended by the Project Bioshield Act of 2004 (public Law 108-276).
[DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)]
6. Current available formulations/shelf life
Formulation
-
Composition of RSDL:
Methoxy Polyethylene Glycol 550. CAS# 9004-74-4. 60 – 100% of RSDL. (Annotated by CHEMM to add PubChem information for this CAS#: )
KBDO (Potassium 2,3-butanedione Monoximate). CAS# 103411-45-6. 10 – 30% of RSDL. (Annotated by CHEMM to add that there are no PubChem records for this CAS#) DAM (2,3-Butanedione Monoxime). CAS# 57-71-6. 0.58% of RSDL. (Annotated by CHEMM to add PubChem information for this CAS#: https://pubchem.ncbi.nlm.nih.gov/#query=57-71-6
RSDL® (Reactive Skin Decontamination Lotion Kit). Safety Data Sheet 2017.
https://www.qckslvr.com/Images/quicksilveranalytics/sds/_T__RSDL_-_Emergent_PN_10051.pdf(This 2017 Safety Data Sheet is noted but not linked to in Emergent BioSolutions Inc. RSDL Consumer website: https://rsdlready.com/ -
Excerpt:
RSDL:
- Patented and made by Canadian specialists and produced by E-Z-EM, Inc., Lake Success, USA.
- Decontaminates the contaminated skin with tabacco (GA), sarine (GB), soman (GD), cyclohexyl sarine (GF), VX, iperite (HD) and T-2 toxin.
- It is composed of Dekon 139 (an unknown potassium salt) and a small amount of 2,3 butadiene monoxime (DAM), dissolved in a solvent composed of polyethylene glycol monomethyl ether (MPEG) and water.
- Decontamination is accomplished by removal, hydrolysis and nucleophilic substitution. It is not used for prophylactic purposes.
- The lotion is pre-impregnated in a sponge applicator, each applicator being packed in a sealed package.[34]
Pulpea, D., Bunea, M., Rotariu, T., Ginghină, R., Toader, G., Moldovan, A. And Pulpea, B., 2019. Review of materials and technologies used for chemical and radiological decontamination. Journal of Military Technology Vol, 2(1). Review of Materials and Technologies Used for Chemical and Radiological Decontamination
“RSDL has been approved as a medical device, a lotion-impregnated sponge, for the decontamination of skin exposed to chemical warfare agents.”
And
“The volume of the RSDL Kit within each of the 42mL green packet format is enough to decontaminate the average equivalent of the forearms, hands, neck, face, and inside surface of the respirator.
The volume of the RSDL Kit within each of the 21mL green packet format is enough to decontaminate the average equivalent of the hands, neck, face, and inside surface of the respirator, if you’re wearing one.”
https://www.rsdl.com/resources/ (Emergent BioSolutions Inc. web site “for professional audiences in the U.S.”)
-
Formulation
Instructions for Use:
- Identify area of possible contamination.
- Tear open packet quickly at any notch.
- Remove the sponge and wipe the affected area.
- Rinse your hand with water when time permits.
Intended Use:
The Reactive Skin Decontamination Lotion Kit (RSDL) is intended to remove and/or neutralize chemical warfare agents (CWA) and T-2 toxin from the skin. For external use only. Use only if chemical warfare agent exposure is suspected. Do not use if packet seal is compromised.Important Safety Information:
CAUTION: Use only as directed. RSDL is not a substitute for proper protective breathing devices and garments. Not for use prior to exposure (i.e., prophylactic use) or whole-body decontamination. Allow RSDL to remain on the skin for at least two minutes. An RSDL ingredient is absorbed through the skin which may cause adverse effects. Avoid leaving on the skin for long periods. Avoid contact with the eyes and mucous membranes. Remove RSDL with water when conditions permit. Keep out of the reach of children.https://www.rsdl.com/about-rsdl/ (Emergent BioSolutions Inc. web site “for professional audiences in the U.S.”)
-
Usage:
The full strength solution is applied on body surfaces after exposure to chemical warfare agents (RSDL should not be used before exposure since its effectiveness following prophylactic use has not been evaluated). Generally, one 21 mL packet is sufficient to decontaminate hands, neck, and face. The packaging and sponge should be discarded after single use. -
How Supplied:
The FDA has cleared the use of RSDL in 21 and 42 mL packets. Each ml of solution contains 1.25 M or 173 mg of Dekon 139 and 35-43 mg of DAM in an MPEG and water solvent.https://www.rsdl.com/(Emergent BioSolutions Inc. web site “for professional audiences in the U.S.”)
-
Abstract. The objective of this study was to determine whether the effectiveness of Reactive Skin Decontamination Lotion (RSDL) as an immediate decontamination (DC) product following cutaneous exposure to VX was affected by the DC procedure. Fur-clipped, male, unanesthetized guinea pigs were used as subjects. Neat, liquid VX was applied to the clipped skin of the left flank. Two minutes after VX application, the exposure site was decontaminated with one of four RSDL DC procedures: 1) RSDL was applied and left on the skin; 2) RSDL was applied and removed after two min; 3) RSDL was applied, removed after two minutes, reapplied by placing an RSDL pad directly on the exposure site for ten minutes; and 4) RSDL was applied, removed after two minutes, and reapplied by dabbing the exposure site once with a fresh RSDL pad. Dose-lethality curves for VX were established for the RSDL DC procedures based on 24-hour alive-or-dead responses. LD50s of VX were calculated by probit analysis. RSDL DC procedures 1, 2 and 3 resulted in VX LD50s that were similar, while procedure 4 resulted in a VX LD50 that was significantly higher than each of the other three VX LD50s. The results suggest that the effectiveness of RSDL as a skin DC product is dependent on the DC procedure, with physical removal and reapplication being the most effective.
USAMRICD-TR-17-01 Comparison of Four Skin Decontamination Procedures Using Reactive Skin Decontamination Lotion {RSDL) Following Cutaneous VX Exposure in Guinea Pigs.
Irwin Koplovitz, Susan Schulz, Julia Morgan, Robert Reed, Edward Clarkson, C. Gary Hurst. January 2016 Approved for public release; distribution unlimited US Army Medical Research Institute of Chemical Defense, 2900 Ricketts Point Road, Aberdeen Proving Ground, MD 21010-5400. An element of the US Army Medical Research and Materiel Command
https://apps.dtic.mil/sti/pdfs/AD1025152.pdf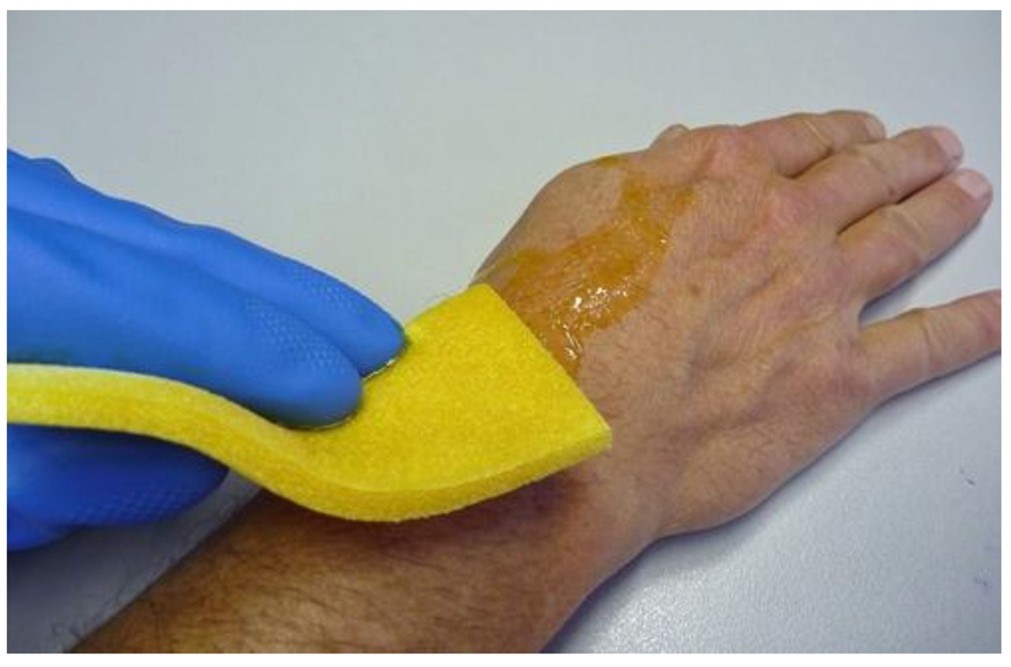
Figure 3: “Skin decontamination by RSDL sponge https://www.mdpi.com/2305-6304/2/2/307#
Capoun, T.; Krykorkova, J. Comparison of Selected Methods for Individual Decontamination of Chemical Warfare Agents. Toxics 2014, 2, 307-326.
https://www.mdpi.com/2305-6304/2/2/307
Shelf life
Stability
-
“The shelf life of an unopened packet of the RSDL Kit is 5 years from date of manufacture, when stored under recommended storage conditions. Expiration dating and storage recommendations are included on the packaging for the RSDL Kit. The RSDL Kit should be stored between 15°C and 30°C (59°F to 86°F) in the original container, protected from direct sunlight in a dry, cool, and well-ventilated area, away from incompatible materials and food and drink. Keep container unopened and sealed until ready for use.”
https://www.rsdl.com/resources/ (Emergent BioSolutions Inc. web site “for professional audiences in the U.S.”)
-
The RSDL vehicle (MPEG) when combined with some commonly used decontamination materials, i.e., solid powdered HTH (calcium hypochlorite) or solid powdered Super Tropical Bleach causes spontaneous combustion. Should RSDL be used on the same decontamination line as either of these products, care must be taken to keep them apart.
-
Do not discard RSDL packaging and sponge into containers that contain or have contained HTH or Super Tropical Bleach.
RSDL® (Reactive Skin Decontamination Lotion Kit). Safety Data Sheet 2017.
https://www.qckslvr.com/Images/quicksilveranalytics/sds/_T__RSDL_-_Emergent_PN_10051.pdf(This 2017 Safety Data Sheet is noted but not linked to in Emergent BioSolutions Inc. RSDL Consumer website: https://rsdlready.com/ -
Abstract. Reactive Skin Decontamination Lotion (RSDL®) is used for the decontamination of Chemical Warfare Agents and Toxic Industrial Compounds after dermal exposure. It has to be stockpiled over a long period and is handled in all climatic zones. Therefore stability is an essential matter of concern. In this work we describe a study to the chemical stability of RSDL® as basis for an estimation of shelf life. We analysed RSDL® for the active ingredient 2,3-butandione monoxime (diacetylmonooxime, DAM), the putative degradation product dimethylglyoxime (DMG) and unknown degradation products by means of a reversed phase high pressure liquid chromatography (HPLC). Calculations were done according to the Arrhenius equation. Based on the temperature dependent rate constants, the time span was calculated, until defined threshold values for DAM and DMG subject to specification and valid regulations were exceeded. The calculated data were compared to the ones gathered from stockpiled samples and samples exposed during foreign mission. The decline of DAM followed first order kinetics, while formation of DMG could be described by zero order kinetics. The rate constants were distinctively temperature dependent. Calculated data were in good accordance to the measured ones from stockpile and mission. Based on a specified acceptable DAM-content of 90% and a valid threshold value of 0.1% (w/w) for the degradation product DMG, RSDL® proved to be stable for at least four years if stored at the recommended conditions of 15°C-30°C. If continuously stored at higher temperatures shelf life will decrease markedly. Therefore RSDL® is an object for risk orientated quality monitoring during storage.
Bogan R, Maas HJ, Zimmermann T. Chemical stability of reactive skin decontamination lotion (RSDL®). Toxicol Lett. 2018 Sep 1;293:264-268.
https://pubmed.ncbi.nlm.nih.gov/28964811/[PubMed Citation]Clarkson, E.D., Morgan, J.E., Chen, P., Schultz, S.M., Soni, S.D., Capacio, B.R. and Koplovitz, I. (2018), Reactive Skin Decontamination Lotion (RSDL) Maintains Its Effectiveness against Cutaneous Applications of VX Nerve Agent for at Least 5 Years Past Its Expiration Date. The FASEB Journal, 32: 691.2-691.2. Not in PubMed but free full text at: https://faseb.onlinelibrary.wiley.com/doi/10.1096/fasebj.2018.32.1_supplement.691.2
Storage
-
“The storage temperature for the RSDL Kit is between 15°C and 30°C (59°F to 86°F) in the original unopened container, protected from direct sunlight in a dry, cool and well-ventilated area, away from incompatible materials and food and drink. Keep container unopened and sealed until ready for use.”
And
“The RSDL Kit has a WARNING: Fire Hazard. Combustion may occur upon contact with strong oxidizing chemicals (e.g., HTH [calcium hypochlorite], Super Tropical Bleach). Do not discard used RSDL components into strong oxidizing chemicals or their containers.”
https://www.rsdl.com/resources/ (Emergent BioSolutions Inc. web site “for professional audiences in the U.S.”)
-
The RSDL vehicle (MPEG) when combined with some commonly used decontamination materials, i.e., solid powdered HTH (calcium hypochlorite) or solid powdered Super Tropical Bleach causes spontaneous combustion. Should RSDL be used on the same decontamination line as either of these products, care must be taken to keep them apart.
-
Do not discard RSDL packaging and sponge into containers that contain or have contained HTH or Super Tropical Bleach.
RSDL® (Reactive Skin Decontamination Lotion Kit). Safety Data Sheet 2017.
https://www.qckslvr.com/Images/quicksilveranalytics/sds/_T__RSDL_-_Emergent_PN_10051.pdf(This 2017 Safety Data Sheet is noted but not linked to in Emergent BioSolutions Inc. RSDL Consumer website: https://rsdlready.com/
https://chemm.hhs.gov/countermeasure_RSDL.htm
7. Current off label utilization and dosing
— including children-, pregnancy-, geriatric-, and obesity-related data8. Route of Administration/Monitoring
-
“Use only as directed. Not for use prior to exposure (i.e., prophylactic use) or whole-body decontamination. Allow RSDL to remain on the skin for at least two minutes. An RSDL ingredient is absorbed through the skin and may cause adverse effects. Avoid leaving on skin for long periods. Avoid contact with the eyes. Remove RSDL when conditions permit.”
And -
“It is recommended that the RSDL be left on the exposed skin for a minimum of 2 minutes then remove when conditions permit. Soap and water can be used to remove the RSDL Kit when conditions permit.”
-
Avoid extended contact with the skin. In emergency conditions, does not require immediate removal from the skin and can be left on under protective clothing, but should be rinsed as soon as it is safe to do so. One of the ingredients (DAM) is absorbed through the skin. RSDL has not been tested in humans using amounts required for decontamination. Intravenous injections of DAM have been shown to cause serious systemic toxicity up to and including a transient comatose state (unconsciousness). There have been no instances of death. Topical adverse effects are not expected to be nearly as severe as with intravenous administration. Pending further studies, do not use RSDL for whole body decontamination or use excessive quantities.
(Emergent BioSolutions Inc. web site “for professional audiences in the U.S.”)
9. Adverse effects
-
“Can the use of the RSDL Kit result in adverse reactions?
IMPORTANT SAFETY INFORMATION that you should be aware of is:
RSDL is for external use only.
Use only if chemical warfare agent exposure is suspected. -
The RSDL Kit has a CAUTION statement: Use only as directed. RSDL is not a substitute for proper protective breathing devices and garments. Not for use prior to exposure (i.e., prophylactic use) or whole-body decontamination. Allow RSDL to remain on the skin for at least two minutes. An RSDL ingredient is absorbed through the skin which may cause adverse effects. Avoid leaving on the skin for long periods. Avoid contact with the eyes and mucous membranes. Remove RSDL with water when conditions permit. Keep out of the reach of children.
The RSDL Kit also has a WARNING: Fire Hazard. Combustion may occur upon contact with strong oxidizing chemicals (e.g., HTH [calcium hypochlorite], Super Tropical Bleach). Do not discard used RSDL components into strong oxidizing chemicals or their containers.”
(Emergent BioSolutions Inc. web site “for professional audiences in the U.S.”)
Also see: RSDL® (Reactive Skin Decontamination Lotion Kit). Safety Data Sheet 2017.
https://www.qckslvr.com/Images/quicksilveranalytics/sds/_T__RSDL_-_Emergent_PN_10051.pdf(This 2017 Safety Data Sheet is noted but not linked to in Emergent BioSolutions Inc. RSDL Consumer website: https://rsdlready.com/
10. Contraindication(s)
-
RSDL should not be used for wound decontamination because its effects on wounds and effects resulting from its absorption through the wound have not been studied.
https://www.rsdl.com/(Emergent BioSolutions Inc. web site “for professional audiences in the U.S.”)
11. Clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issuesNo data available at this time.
12. Non-clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issuesNo data available at this time.
13. Needed studies for Chemical Defense clinical indication
— including pharmacokinetics, safety, efficacy, pregnancy, breastfeeding, and review panel recommendationsNo data available at this time.
14. Needed studies for non Chemical Defense clinical indications
— including review panel recommendations-
Abstract Although skin decontamination has been studied since WWI, there has yet been an optimal protocol for determining in vitro skin decontamination efficacy due to the complexity of percutaneous absorption. Here we explore methods of quantifying decontamination through the parameters of percutaneous absorption in different models: humans, animals, in vitro assays, and computer modeling, with humans being an ideal model, followed by rhesus monkeys, weanling pigs, hairless guinea pigs, and hairless rats. In vitro assays are also useful, but skin type/thickness, receptor fluid, etc. must be chosen carefully as these can influence absorption. Computer modeling provides another way to quantify percutaneous absorption when sufficient input data becomes available. Reactive skin decontamination lotion (RSDL) is considered a gold standard for decontamination and is used by the military to decontaminate chemical warfare agents. Although there is evidence that supports RSDL superiority over other methods of decontamination, lack of in vivo human studies and diversity in types of compounds tested remain a data limitation. An ideal decontamination method is applying the contaminant on the test model and measuring the mass absorbed when a decontaminant is used compared to when no decontaminant is used. A perfect decontaminant would result in no contaminant absorption.
Tran, T., Maibach, H.I. (2022). Toward a Harmonized Protocol for Quantifying In Vitro Human Skin Decontamination Efficacy. In: Feschuk, A.M., Law, R.M., Maibach, H.I. (eds) Dermal Absorption and Decontamination. Springer, Cham.
https://link.springer.com/chapter/10.1007/978-3-031-09222-0_2
15. Study-related ethical concerns
— including review panel recommendationsNo data available at this time.
16. Global regulatory status
Procured or Recognized by:
- US Department of Defense
- Joint Program Executive Office for Chemical and Biological Defense
- Other military services since 2002
Certified by:
- US Department of Homeland Security, SAFETY Act
Cleared by:
- US Food and Drug Administration
- Health Canada
- Australian Therapeutics Goods Administration
- Israel Ministry of Health
- RSDL Kit is CE-marked
(Emergent BioSolutions Inc. web site “for professional audiences in the U.S.”)
U.S.
The Food and Drug Administration (FDA) has cleared for use by the U.S. military a liquid decontamination lotion intended to remove or neutralize chemical warfare agents and T-2 fungal toxin from the skin. The lotion, called Reactive Skin Decontamination Lotion (RSDL), must be applied to exposed skin as soon as possible after exposure to a chemical agent.
FDA Drugs: FDA Clears Skin Lotion for Military to Protect Against Chemical Burns. March 28, 2003 (FDA)
https://chemm.hhs.gov/countermeasure_RSDL.htm
17. Other potentially useful information
-
Working dogs are widely used by service professionals and the military for diverse roles that include sentry, patrol, messenger, tracking, search and rescue, law enforcement, apprehension, as well as explosives and narcotics detection. The expected tasks performed are in many ways determined by the breed, which is customarily a German Shepherd, Dutch Shepherd, Golden Retriever, Border Collie, Labrador Retriever, Beagle, or Belgium Malinois. Working dogs may be subject to injury from dangerous work environments or harmful agent exposure. Personal protective equipment (PPE) has been developed for such dogs, but may impede performance of duties or be poorly tolerated. Canine-specific field-use ready decontamination techniques and kits are therefore needed for use on working dogs that have encountered a harmful agent exposure. This report briefly reviews the development of the military working dog and examines personal protective equipment and decontamination techniques for working dogs after exposure to harmful biologic or chemical agents.
And
In 2003, the FDA approved RSDL®, a liquid skin decontamination lotion that neutralizes vesicant chemical or organophosphorus nerve agents (28). RSDL® is currently supplied to all branches of the military for topical human and canine use (13,29 ).
Protection of MWDs in a CBRN (“chemical, biological, radiological, nuclear”) environment is a high priority that is acknowledged to be difficult (13). The preferred method of decontaminating MWDs exposed to nerve agents is by first using Reactive Skin Decontamination Lotion (RSDL®), followed by thoroughly washing and rinsing the MWD to ensure all contaminants are removed (13). MWDs exposed to vesicants (blister agents) are decontaminated with thorough washing and rinsing, with the exception of sulfur mustard (H/HD), in which case only RSDL® should be used because “wet skin absorbs more agent than does dry skin”(13). MWD sensitivity to nerve and blister agents varies based on the agent and the exposure form (inhalation vs. dermal exposure); however, in many instances these dogs are less sensitive than their human partner (14). Decreased sensitivity does not equal zero morbidity and contaminated individuals are a potential hazard to the individuals they come in contact with.
And
In summary, the use of PPE for canines is often impractical; shampooing with copious rinsing is not without logistical challenges, may increase percutaneous absorption, and should be limited in frequency; and current decontamination products were developed for humans. It is logical to assume that evidence-based canine decontamination protocols are underrepresented in the veterinary literature (21)because they are underrepresented in research and development. Thus, further research is warranted to establish proven chemical class-specific treatment protocols that will effectively decontaminate working dogs following exposures in the field, protecting not only the dogs but their handlers.
(Annotation. Reference 28 is Reactive Skin Decontamination Lotion (RSDL)-Medical Countermeasures Database. (2017). Available at: https://chemm.nlm.nih.gov/countermeasure_RSDL.htm (accessed July 15, 2021). This is an old/broken link.)
Jarrett CL, Brathwaite M, Gogal RM, Holladay SD. Working Dog Service, Harmful Agent Exposure and Decontamination. Front Vet Sci. 2022 May 2;9:892998. [PubMed Citation]
Abstract. Purpose: This study investigated the decontamination effectiveness of selected toxic industrial chemicals using RSDL® (Reactive Skin Decontamination Lotion Kit; Emergent BioSolutions Inc.; https://www.rsdl.com/). Materials and methods: Quantitative analytical methods were developed for dermal toxic compounds of varying physicochemical properties: sulfuric acid, hydrofluoric acid, ammonia, methylamine, hydrazine, phenylhydrazine, 1,2-dibromoethane, capsaicin, and fentanyl. These methods were subsequently used to evaluate the decontamination effectiveness on painted metal substrates at an initial chemical contamination level of 10g/m2 (0.1g/m2 for fentanyl). Results: The decontamination effectiveness ranged from 97.79% to 99.99%. Discussion and conclusion: This study indicates that the RSDL kit may be amenable for use as an effective decontaminant for material substrates beyond the classical chemical warfare agents and the analytical methods may be used for future decontamination assessment studies using contaminated skin or other materials.
Verheij ER, Joosen MJA, Cochrane L, de Bruin-Hoegee M, de Koning MC. Decontamination of Toxic Industrial Chemicals and Fentanyl by Application of the RSDL® Kit. J Spec Oper Med. 2020 Spring;20(1):55-59.
https://pubmed.ncbi.nlm.nih.gov/32203607/[PubMed Citation]“RSDL was initially developed by Defence Research and Development Canada, an agency of the Canadian Department of National Defence (DND), to prepare the Canadian forces for chemical warfare attacks. The first configuration of RSDL was distributed to Canadian troops during the First Gulf War in 1991. The U.S. Department of Defense (DOD) became interested in RSDL and began testing on the product after the Gulf War. The DOD then filed to the U.S. Food and Drug Administration (FDA) which issued 510(k) clearance for RSDL in November 2002. The European CE Mark, Australian TGA clearance and Israeli Ministry of Health clearances were later issued. RSDL has been adopted by several militaries around the world since that time. Since its development, the RSDL Kit has been sold in over 35 countries.”
https://www.rsdl.com/about-rsdl/(Emergent BioSolutions Inc. web site “for professional audiences in the U.S.”)Abstract. The study examined the degradation of riot control agents (RCAs): 2-chloroacetophenone (CN), 2-chlorobenzalmalononitrile (CS), and capsaicin, using the Reactive Skin Decontamination Lotion Kit (RSDL®) lotion and evaluated the direct liquid phase reactivity of the RSDL lotion component with each RCA. RSDL lotion was mixed with the selected RCAs at different molar ratios. Reactivity of the active ingredient potassium 2,3-butanedione monoximate (KBDO) with the RCA was observed for one hour. Samples of 10 μL were taken and quenched, analyzed for residual RCA using LC-MS. CN, was degraded at molar ratios of two and above in less than 2 min. At a molar ratio of 1:1 KBDO:CN, ∼90 % of CN was degraded within 2 min, the remaining 10 % residual CN was observed for one hour without any change. CS, degradation of more than 68 % of CS was achieved at 20:1 M ratio of KBDO:CS within 1 h of reaction time. For capsaicin, no degradation was observed regardless of the higher molar ratios of up to 20:1 and longer reaction times of up to one hour. This study provides evaluation of neutralizing action of the RSDL lotion without assessment of the physical removal component by the RSDL Kit.
Gebremedhin M, Fentabil M, Cochrane L, Lau V, Toth D, Barry J. In vitro decontamination efficacy of the RSDL® (Reactive Skin Decontamination Lotion Kit) lotion component against riot control agents: Capsaicin, Mace™ (CN) and CS. Toxicol Lett. 2020 Oct 10;332:36-41.
https://pubmed.ncbi.nlm.nih.gov/32629075/[PubMed Citation]Abstract. Ricin is a proteinaceous toxin, listed on the schedules of both the chemical and biological weapons conventions. The ease of accessibility to the Ricinus communis plant and toxin extraction makes ricin a viable concern for use of intentional release and causal effects. The adverse effects following exposure to the toxin are caused by the bipartite molecular structure of ricin which allows binding to the mammalian cell surface, enter via endocytic uptake, and deliver the catalytically active polypeptide into the cell cytosol where it irreversibly inhibits protein synthesis, causing cell death. In the present study, the inactivation effectiveness of RSDL® (Reactive Skin Decontamination Lotion) and its individual inactivating constituents (Potassium 2,3-butanedione monoximate (KBDO) and 2,3-butanedione (DAM)) was evaluated for ricin using a number of read out systems including a cytotoxicity assay, quantitative sandwich ELISA test, and a mass spectrometry-based assay. The results demonstrate that RSDL is able to abolish ricin activity after an incubation time of 30 min as determined in the cytotoxicity assay, and after 2 min as determined in the ELISA assay. Mass spectrometric analysis provided evidence that RSDL is able to induce cleavage of the disulfide linkage between the A- and B- polypeptide chain of ricin which is crucial to the inactivation of the toxin, but this seems not the only mechanism of inactivation. Follow on studies would assist to elucidate the details of the toxin inactivation because it is possible that additional generic mechanisms are in place for denaturation with the RSDL lotion components. This may also provide a promise for testing and inactivation with RSDL of other protein toxins.
van den Berg RM, Joosen MJA, Savransky V, Cochrane L, Noort D. Inactivation of ricin by constituents present in a skin decontamination lotion. Chem Biol Interact. 2022 Sep 25;365:110055.
https://pubmed.ncbi.nlm.nih.gov/35963314/ [PubMed Citation]
18. Publications
Bide RW, Schofield L and Risk DJ. Immediate post-dosing paralysis following severe Soman and VX toxicosis in guinea pigs. J Appl Toxicol. 2005 Sep-Oct;25(5):410-7. [PubMed Citation] (Not in TRACIE.)
Bjarnason S, Mikler J, Hill I, Tenn C, Garrett M, Caddy N and Sawyer TW. Comparison of selected skin decontaminant products and regimens against VX in domestic swine. Hum Exp Toxicol. 2008 Mar;27(3):253-61. [PubMed Citation](Not in TRACIE.)
Bogan R, Maas HJ, Zimmermann T. Chemical stability of reactive skin decontamination lotion (RSDL®). Toxicol Lett. 2018 Sep 1;293:264-268. https://pubmed.ncbi.nlm.nih.gov/28964811/ [PubMed Citation] (Not in TRACIE.)
Braue EH Jr, Smith KH, Doxzon BF, Lumpkin HL, Clarkson ED. Efficacy studies of Reactive Skin Decontamination Lotion, M291 Skin Decontamination Kit, 0.5% bleach, 1% soapy water, and Skin Exposure Reduction Paste Against Chemical Warfare Agents, part 1: guinea pigs challenged with VX. Cutan Ocul Toxicol. 2011 Mar;30(1):15-28 [PubMed Citation](Not in TRACIE.)
Braue EH Jr, Smith KH, Doxzon BF, Lumpkin HL, Clarkson ED. Efficacy studies of Reactive Skin Decontamination Lotion, M291 Skin Decontamination Kit, 0.5% bleach, 1% soapy water, and Skin Exposure Reduction Paste Against Chemical Warfare Agents, part 1: guinea pigs challenged with VX. Cutan Ocul Toxicol. 2011 Mar;30(1):15-28 [PubMed Citation] (Not in TRACIE.)
Braue EH Jr, Smith KH, Doxzon BF, Lumpkin HL, Clarkson ED. Efficacy studies of Reactive Skin Decontamination Lotion, M291 Skin Decontamination Kit, 0.5% bleach, 1% soapy water, and Skin Exposure Reduction Paste Against Chemical Warfare Agents, Part 2: Guinea pigs challenged with soman Cutan Ocul Toxicol. 2011 Mar;30(1):29-37. [PubMed Citation] (Not in TRACIE.)
Cao Y, Hui X, Elmahdy A, Maibach H. In vitro human skin permeation and decontamination of diisopropyl methylphosphonate (DIMP) using Dermal Decontamination Gel (DDGel) and Reactive Skin Decontamination Lotion (RSDL) at different timepoints. Toxicol Lett. 2018 Dec 15;299:118-123. https://pubmed.ncbi.nlm.nih.gov/30282006/[PubMed Citation] (Not in TRACIE.)
Capoun, T.; Krykorkova, J. Comparison of Selected Methods for Individual Decontamination of Chemical Warfare Agents. Toxics 2014, 2, 307-326. https://www.mdpi.com/2305-6304/2/2/307 (Not in TRACIE.)
Clarkson, E.D., Morgan, J.E., Chen, P., Schultz, S.M., Soni, S.D., Capacio, B.R. and Koplovitz, I. (2018), Reactive Skin Decontamination Lotion (RSDL) Maintains Its Effectiveness against Cutaneous Applications of VX Nerve Agent for at Least 5 Years Past Its Expiration Date. The FASEB Journal, 32: 691.2-691.2. Not in PubMed but free full text at: https://faseb.onlinelibrary.wiley.com/doi/10.1096/fasebj.2018.32.1_supplement.691.2 (Not in TRACIE.)
Collins, S., James, T., Southworth, F. et al. Human volunteer study of the decontamination of chemically contaminated hair and the consequences for systemic exposure. Sci Rep 10, 20822 (2020). https://www.nature.com/articles/s41598-020-77930-1 (Not in TRACIE.)
And
Collins, S., James, T., Southworth, F. et al. Author Correction: Human volunteer study of the decontamination of chemically contaminated hair and the consequences for systemic exposure. Sci Rep 11, 9070 (2021). (“This Article contains errors in Figure 2.”) https://www.nature.com/articles/s41598-021-88761-z (Not in TRACIE.)
Connolly JM, Stevenson RS, Railer RF, Clark OE, Whitten KA, Lee-Stubbs RB, Anderson DR. Impairment of wound healing by reactive skin decontamination lotion (RSDL®) in a Göttingen minipig® model. Cutan Ocul Toxicol. 2020 Jun;39(2):143-157. https://pubmed.ncbi.nlm.nih.gov/32321319/ [PubMed Citation] (Not in TRACIE.)
Dachir S, Cohen M, Buch H, Kadar T. Skin decontamination efficacy of sulfur mustard and VX in the pig model: A comparison between Fuller's earth and RSDL. Chem Biol Interact. 2021 Feb 25;336:109393. https://pubmed.ncbi.nlm.nih.gov/33508307/ [PubMed Citation] (Not in TRACIE.)
Duncan S, Mikler J, Jackson Lepage C, Clewley R, Boulay Greene H. Responding to an incident involving organophosphorus nerve agents Safety advisory and guidance. Defence Research and Development. Canada Reference Document DRDC-RDDC-2021-D106. September 2021. https://cradpdf.drdc-rddc.gc.ca/PDFS/unc366/p813529_A1b.pdf(Not in TRACIE.)
[DHHS/FDA; Emergency Preparedness and Response- Counterterrorism and Emerging Threats (12/01/2011)]
FDA Drugs: FDA Clears Skin Lotion for Military to Protect Against Chemical Burns. March 28, 2003 (FDA)
Fentabil M, Gebremedhin M, Barry J, Mikler J, Cochrane L. In vivo efficacy of the Reactive Skin Decontamination Lotion (RSDL®) kit against organophosphate and carbamate pesticides. Chem Biol Interact. 2020 Feb 25;318:108980. https://pubmed.ncbi.nlm.nih.gov/32044340/ [PubMed Citation] (Not in TRACIE.)
Fentabil M, Gebremedhin M, Purdon JG, Cochrane L, Goldman VS. Degradation of pesticides with RSDL® (reactive skin decontamination lotion kit) lotion: LC-MS investigation. Toxicol Lett. 2018 Sep 1;293:241-248. https://pubmed.ncbi.nlm.nih.gov/29128639/[PubMed Citation] (Not in TRACIE.)
Feschuk AM, Law RM, Maibach HI. Comparative efficacy of Reactive Skin Decontamination Lotion (RSDL): A systematic review. Toxicol Lett. 2021 Oct 1;349:109-114. https://pubmed.ncbi.nlm.nih.gov/34147606/ [PubMed Citation] (Not in TRACIE.)
Feschuk, A.M., Law, R.M., Maibach, H.I. (2022). A Review of Reactive Skin Decontamination Lotion Efficacy. In: Feschuk, A.M., Law, R.M., Maibach, H.I. (eds) Dermal Absorption and Decontamination. Springer, Cham. https://link.springer.com/chapter/10.1007/978-3-031-09222-0_8 (Not in TRACIE.)
Gebremedhin M, Fentabil M, Cochrane L, Lau V, Toth D, Barry J. In vitro decontamination efficacy of the RSDL® (Reactive Skin Decontamination Lotion Kit) lotion component against riot control agents: Capsaicin, Mace™ (CN) and CS. Toxicol Lett. 2020 Oct 10;332:36-41. https://pubmed.ncbi.nlm.nih.gov/32629075/[PubMed Citation] (Not in TRACIE.)
Hayoun MA, Smith ME, Ausman C, et al. Toxicology, V-Series Nerve Agents. [Updated 2022 Sep 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441997/ (Not in TRACIE.)
Jarrett CL, Brathwaite M, Gogal RM, Holladay SD. Working Dog Service, Harmful Agent Exposure and Decontamination. Front Vet Sci. 2022 May 2;9:892998. https://pubmed.ncbi.nlm.nih.gov/35585862/[PubMed Citation] (Not in TRACIE.)
Johnston GM, Wills BK. Chemical Decontamination. [Updated 2022 Jun 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538161/(Not in TRACIE.)
Joosen MJ, van der Schans MJ, Kuijpers WC, van Helden HP, Noort D. Timing of decontamination and treatment in case of percutaneous VX poisoning: a mini review. Chem Biol Interact. 2013 Mar 25;203(1):149-53. [PubMed Citation] (Not in TRACIE)
Josse D, Wartelle J, Cruz C. Showering effectiveness for human hair decontamination of the nerve agent VX. Chem Biol Interact2015; 232:94-100. [PubMed Citation] (Not in TRACIE.)
MacIntyre, C.R., Chughtai, A.A., Bhattacharjee, S., Kunasekaran, M.P. and Engalls, T., 2020. Risk mitigation of inadvertent exposure to biothreats to front line law enforcement. Global Biosecurity, 2(1), p. None. https://jglobalbiosecurity.com/articles/10.31646/gbio.59/ (Not in TRACIE.)
Madsen,. Chemical terrorism: Rapid recognition and initial medical management. UpToDate. https://www.uptodate.com/contents/chemical-terrorism-rapid-recognition-and-initial-medical-management (In TRACIE as 2019 “Date Published.”)
Mikler J, Tenn C, Worek F, Reiter G, Thiermann H, Garrett M, Bohnert S, Sawyer TW. Immobilization of Russian VX skin depots by localized cooling: Implications for decontamination and medical countermeasures. Toxicol Lett. 2011 Sep 25;206(1):47-53. [PubMed Citation]
Oudejans L, O’Kelly J, Evans AS, Wyrzykowska-Ceradini B, Abderrahmane Touati A, Dennis Tabor D, Snyder EG,
Decontamination of personal protective equipment and related materials contaminated with toxic industrial chemicals and chemical warfare agent surrogates,
Journal of Environmental Chemical Engineering, Volume 4, Issue 3, 2016, Pages 2745-2753.
https://www.sciencedirect.com/science/article/pii/S2213343716301920 (Not in PubMed) (Not in TRACIE.)Pulpea, D., Bunea, M., Rotariu, T., Ginghină, R., Toader, G., Moldovan, A. And Pulpea, B., 2019. Review of materials and technologies used for chemical and radiological decontamination. Journal of Military Technology Vol, 2(1). Review of Materials and Technologies Used for Chemical and Radiological Decontamination (Not in TRACIE.)
Rolland P, Bolzinger MA, Cruz C, Josse D, Briançon S. Hairy skin exposure to VX in vitro: effectiveness of delayed decontamination. Toxicol In Vitro. 2013 Feb;27(1):358-66. [PubMed Citation]
Roul A, Maibach HHI (2020) Skin Decontamination 2021. Emerg Med Inves 5: 10105. https://www.gavinpublishers.com/article/view/skin-decontamination-2021 (Not in TRACIE.)
Salerno A, Devers T, Bolzinger MA, Pelletier J, Josse D, Briançon S. In vitro skin decontamination of the organophosphorus pesticide Paraoxon with nanometric cerium oxide CeO2.Chem Biol Interact. 2017; 267:57-66. [PubMed Citation] (Not in TRACIE.)
Sawyer TW, Mikler J, Worek F, Reiter G, Thiermann H, Tenn C, Weatherby K, Bohnert S. The therapeutic use of localized cooling in the treatment of VX poisoning. Toxicol Lett. 2011 Jul 4;204(1):52-6. [PubMed Citation]
Schwartz MD, Hurst CG, Kirk MA, Reedy SJ, Braue EH. Reactive Skin Decontamination Lotion (RSDL) for the Decontamination of Chemical Warfare Agent (CWA) Dermal Exposure. Curr Pharm Biotechnol. 2012 Aug 1;13(10):1971-9. [PubMed Citation]
Spiandore M, Piram A, Lacoste A, Prevost P, Maloni P, Torre F, Asia L, Josse D, Doumenq P. Efficacy of scalp hair decontamination following exposure to vapours of sulphur mustard simulants 2-chloroethyl ethyl sulphide and methyl salicylate. Chem Biol Interact. 2017; 267:74-79. [PubMed Citation] (Not in TRACIE.)
Taysse L, Daulon S, Delamanche S, Bellier B and Breton P. Skin decontamination of mustards and organophosphates: comparative efficiency of RSDL and Fuller's earth in domestic swine. Hum Exp Toxicol. 2007 Feb;26(2):135-41. [PubMed Citation]
Taysse L, Dorandeu F, Daulon S, Foquin A, Perrier N, Lallement G and Breton P. Cutaneous challenge with chemical warfare agents in the SKH-1 hairless mouse (II): Effects of some currently used skin decontaminants (RSDL and Fuller's earth) against liquid sulphur mustard and VX exposure. Hum Exp Toxicol. 2011 Jun;30(6):491-8. [PubMed Citation]
Thiermann H, Aurbek N, and Worek F, CHAPTER 1: Treatment of Nerve Agent Poisoning , in Chemical Warfare Toxicology: Volume 2: Management of Poisoning, 2016, pp. 1-42. https://pubs.rsc.org/en/content/chapterhtml/2016/bk9781782628033-00001 (Not in TRACIE.)
Thors L, Koch M, Wigenstam E, Koch B, Hägglund L, Bucht A. Comparison of skin decontamination efficacy of commercial decontamination products following exposure to VX on human skin Chem Biol Interact. 2017 Aug 1;273:82-89. [PubMed Citation] (Not in TRACIE.)
Thors L, Lindberg S, Johansson S, Koch B, Koch M, Hägglund L, Bucht A. RSDL decontamination of human skin contaminated with the nerve agent VX. Toxicol Lett. 2017 Mar 5;269:47-54. https://pubmed.ncbi.nlm.nih.gov/28179194/ [PubMed Citation] (Not in TRACIE.)
Thors L, Wigenstam E, Qvarnström J, Hägglund L, Bucht A. Improved skin decontamination efficacy for the nerve agent VX. Chem Biol Interact. 2020 Jul 1;325:109-135. https://pubmed.ncbi.nlm.nih.gov/32428449/ [PubMed Citation] (Not in TRACIE.)
Thors L, Wigenstam E, Qvarnström J, Bucht A. Efficient agent degradation within skin is important for decontamination of percutaneously exposed VX. Cutan Ocul Toxicol. 2021 Jun;40(2):95-102. https://pubmed.ncbi.nlm.nih.gov/33759679/ [PubMed Citation] (Not in TRACIE.)
Thors L, Wästerby P, Wigenstam E, Larsson A, Öberg L, Bucht A. Do cold weather temperatures affect the efficacy of skin decontamination? J Appl Toxicol. 2022 Jun;42(6):961-969. https://pubmed.ncbi.nlm.nih.gov/34850419/ [PubMed Citation] (Not in TRACIE.)
Tran, T., Maibach, H.I. (2022). Toward a Harmonized Protocol for Quantifying In Vitro Human Skin Decontamination Efficacy. In: Feschuk, A.M., Law, R.M., Maibach, H.I. (eds) Dermal Absorption and Decontamination. Springer, Cham. https://link.springer.com/chapter/10.1007/978-3-031-09222-0_2 (Not in TRACIE.)
USAMRICD-TR-17-01 Comparison of Four Skin Decontamination Procedures Using Reactive Skin Decontamination Lotion {RSDL) Following Cutaneous VX Exposure in Guinea Pigs.
Irwin Koplovitz, Susan Schulz, Julia Morgan, Robert Reed, Edward Clarkson, C. Gary Hurst. January 2016 Approved for public release; distribution unlimited US Army Medical Research Institute of Chemical Defense, 2900 Ricketts Point Road, Aberdeen Proving Ground, MD 21010-5400. An element of the US Army Medical Research and Materiel Command
https://apps.dtic.mil/sti/pdfs/AD1025152.pdf (Not in TRACIE.)van den Berg RM, Joosen MJA, Savransky V, Cochrane L, Noort D. Inactivation of ricin by constituents present in a skin decontamination lotion. Chem Biol Interact. 2022 Sep 25;365:110055. https://pubmed.ncbi.nlm.nih.gov/35963314/ [PubMed Citation] (Not in TRACIE.)
Verheij ER, Joosen MJA, Cochrane L, de Bruin-Hoegee M, de Koning MC. Decontamination of Toxic Industrial Chemicals and Fentanyl by Application of the RSDL® Kit. J Spec Oper Med. 2020 Spring;20(1):55-59. https://pubmed.ncbi.nlm.nih.gov/32203607/ [PubMed Citation] (Not in TRACIE.)
Walters TJ, Kauvar DS, Reeder J, Baer DG. Effect of Reactive Skin Decontamination Lotion on Skin Wound Healing in Laboratory Rats. Mil Med. 2007 Mar;172(3):318-21. [PubMed Citation] (Not in TRACIE.)
19. Web sites
-
RSDL® (Reactive Skin Decontamination Lotion Kit). Safety Data Sheet 2017.
https://www.qckslvr.com/Images/quicksilveranalytics/sds/_T__RSDL_-_Emergent_PN_10051.pdf(This 2017 Safety Data Sheet is noted but not linked to in Emergent BioSolutions Inc. RSDL Consumer website: https://rsdlready.com/) -
NIH CounterACT Program (HHS/NIH)
-
Consumer Product Information Database (CPID). No RSDL information as of 11/8/22.
-
FDA, What are Medical Countermeasures? https://www.fda.gov/emergency-preparedness-and-response/about-mcmi/what-are-medical-countermeasures
-
FDA Clears Skin Lotion for Military to Protect Against Chemical Burns https://www.fda.gov/drugs/bioterrorism-and-drug-preparedness/fda-clears-skin-lotion-military-protect-against-chemical-burns
-
Products Approved for Chemical Emergencies
https://www.fda.gov/drugs/bioterrorism-and-drug-preparedness/products-approved-chemical-emergencies -
Haz-Map. No information about RSDL as of 11/8/22.
https://aspr.hhs.gov/MCM/Pages/default.aspx -
https://www.phe.gov/preparedness/mcm/phemce/Pages/default.aspx
-
NIH NLM ClinicalTrials.gov
NIH NLM ClinicalTrials.govNo clinical trials of RSDL in this database as of 12/20/22.
-
NIH NLM NCBI PubChem
https://pubchem.ncbi.nlm.nih.gov/RSDL in PubChem consists of PubMed citations and one patent:
https://pubchem.ncbi.nlm.nih.gov/#query=RSDL -
NIH NLM NCBI PubMed
https://pubmed.ncbi.nlm.nih.gov/PubMed search of “RSDL”
https://pubmed.ncbi.nlm.nih.gov/?term=RSDL
(Note: Results needed to be filtered and were since RSDL search also delivers results for “rudimentary successional dental lamina” and “Red seabream digestive lipase.”PubMed RSDL Public Collection as of 11/5/22 (n= 38 selected as relevant and/or of interest out of the original total of 42):
https://pubmed.ncbi.nlm.nih.gov/collections/62336060/?sort=pubdate -
NIH NINDS CounterACT Program. No RSDL content.
https://www.ninds.nih.gov/current-research/trans-agency-activities/counteract-program
-
-
NIH RePORTER
https://reporter.nih.gov/. No RSDL content. -
NIH NLM DailyMed. No RSDL content.
https://dailymed.nlm.nih.gov/dailymed/index.cfm
Record last updated 11/04/2024
In addition to the RSDL record in the CHEMM Medical Countermeasures Database, note the following in CHEMM: (Will update all of these as needed once the updated and enhanced Medical Countermeasures Database record is reviewed and approved during 2023.)
-
(11/13/22 note: This comes from “Decontamination of First Responders” and its “Link to RSDL background information.” Also see below. https://chemm.hhs.gov/appendix10.htm)
-
About RSDL Use (Emergent BioSolutions)
-
FDA Clears Skin Lotion for Military to Protect Against Chemical Burns(FDA) (... The lotion, called Reactive Skin Decontamination Lotion (RSDL), must be applied to exposed skin as soon as possible after exposure to a chemical agent. ... )
-
RSDL Product Resources (RSDL)
-
Braue EH Jr, Smith KH, Doxzon BF, Lumpkin HL, Clarkson ED. Efficacy studies of Reactive Skin Decontamination Lotion, M291 Skin Decontamination Kit, 0.5% bleach, 1% soapy water, and Skin Exposure Reduction Paste Against Chemical Warfare Agents, part 2: guinea pigs challenged with soman.Cutan Ocul Toxicol. 2011 Mar;30(1):29-37. [PubMed Citation]
-
Taysse L, Daulon S, Delamanche S, Bellier B, Breton P.Skin decontamination of mustards and organophosphates: comparative efficiency of RSDL and Fuller's earth in domestic swine.Hum Exp Toxicol. 2007 Feb;26(2):135-41. [PubMed Citation]
-
Walters TJ, Kauvar DS, Reeder J, Baer DG.Effect of reactive skin decontamination lotion on skin wound healing in laboratory rats.Mil Med. 2007 Mar;172(3):318-21. [PubMed Citation]
-
-
Reactive Skin Decontamination Lotion (RSDL) - Medical Countermeasures Database - CHEMM (Emergent BioSolutions)
https://chemm.hhs.gov/countermeasure_RSDL.htm
Decontamination Lotion (RSDL) Reactive Skin Decontamination Lotion (RSDL) - Medical Countermeasures...Reactive Skin Decontamination Lotion (RSDL) 2 ... -
Fuller's Earth - Medical Countermeasures Database - CHEMM
https://chemm.hhs.gov/countermeasure_fullersearth.htm
Reactive Skin Decontamination Lotion RSDL®). Both simulants were recovered from...Reactive Skin Decontamination Lotion (RSDL) 30 min prior to ... -
Sodium Hypochlorite - Medical Countermeasures Database - CHEMM
https://chemm.hhs.gov/countermeasure_sodium-hypochlorite.htm
...following a 5-LD50 challenge) value for RSDL was only 4.0 minutes. Conclusions: Several...the M291 SDK were less effective than RSDL, but still ... -
https://chemm.hhs.gov/appendix10.htm
https://chemm.hhs.gov/appendix10.htm
Reactive Skin Decontamination Lotion (RSDL) - designed to be carried by First Responders...foam sponge in an impermeable packet. RSDL is highly ... -
Fourth Generation Agents Hospital - Medical Management Guidelines - CHEMM
https://chemm.hhs.gov/nerveagents/FGAMMGHospital.htm
...amounts of chemical contamination. If RSDL is available, it is recommended for...specialized products such as soap or RSDL. Avoid using hand sanitizer ... -
Mustard Prehospital Management
https://chemm.hhs.gov/mustard_prehospital_mmg.htm
Reactive Skin Decontamination Lotion (RSDL) - designed to be carried by First Responders...groups of chemical warfare agents. RSDL performed ... -
Medical Countermeasures Database - CHEMM
https://chemm.hhs.gov/medical_countermeasures.htm
N-acetylcysteine Naloxone Obidoxime Pralidoxime RSDL Scopolamine Sodium bicarbonate Sodium -
Nerve Agents Prehospital Management
https://chemm.hhs.gov/na_prehospital_mmg.htm
Reactive Skin Decontamination Lotion (RSDL) - designed to be carried by First Responders...groups of chemical warfare agents. RSDL performed ... -
Mustard Hospital Management
https://chemm.hhs.gov/mustard_hospital_mmg.htm
Reactive Skin Decontamination Lotion (RSDL) - designed to be carried by First Responders...groups of chemical warfare agents. RSDL performed ... -
Site Map - CHEMM
https://chemm.hhs.gov/sitemap.htm
N-acetylcysteine Naloxone Obidoxime Pralidoxime RSDL Scopolamine Sodium bicarbonate Sodium -
Nerve Agents Hospital Management
https://chemm.hhs.gov/na_hospital_mmg.htm
Reactive Skin Decontamination Lotion (RSDL) - designed to be carried by First Responders...groups of chemical warfare agents. RSDL performed ... -
Fourth Generation Agents Pre-hospital - Medical Management Guidelines - CHEMM
https://chemm.hhs.gov/nerveagents/FGAMMGPrehospital.htm
Reactive Skin Decontamination Lotion (RSDL) is available, it is recommended for...specialized products such as soap or RSDL. Avoid using hand ... -
Fourth Generation Agents: Medical Management Guidelines (January 2019)
https://chemm.hhs.gov/nerveagents/FGA_Medical_Management_Guidelines_508.pdf
Reactive Skin Decontamination Lotion (RSDL) is available, it is recommended for...specialized products such as soap or RSDL. · Avoid using hand ... -
Fourth Generation Agents: Reference Guide (January 2019)
https://chemm.hhs.gov/nerveagents/FGA_Reference_Guide_508.pdf
Lotion (RSDL), if available, is recommended for spot decontamination. RSDL cannot...tropical bleach). Do not discard used RSDL components into strong ... -
HI-6 - Medical Countermeasures Database - CHEMM
https://chemm.hhs.gov/countermeasure_hi-6.htm
Reactive Skin Decontaminant Lotion (RSDL) 15 min after VR exposure was completely...treated with oxime or decontaminated with RSDL. Blood analyses ...
Fourth Generation Agents - Reference Guide - CHEMM
https://chemm.hhs.gov/nerveagents/FGAReferenceGuide.htm
Lotion (RSDL), if available, is recommended for spot decontamination. RSDL cannot...tropical bleach). Do not discard used RSDL components into strong ...
